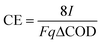Microbial electrochemical systems outperform fixed-bed biofilters in cleaning up urban wastewater†
A.
Aguirre-Sierra‡
a,
T.
Bacchetti-De Gregoris
b,
A.
Berná
b,
J. J.
Salas
c,
C.
Aragón
c and
A.
Esteve-Núñez‡
*ab
aDepartment of Analytical Chemistry and Chemical Engineering, University of Alcalá, E-28805 Alcalá de Henares, Madrid, Spain. E-mail: abraham.esteve@uah.es; Fax: +34 918855088; Tel: +34 918854950
bMadrid Institute for Advanced Studies in Water Technologies IMDEA-Water, Parque Científico Tecnológico, E-28871 Alcalá de Henares, Madrid, Spain
cFoundation Centre for New Water Technologies (CENTA), E-41820 Carrión de los Céspedes, Sevilla, Spain
First published on 15th September 2016
Abstract
In this work, we present for the first time the concept of integrating microbial electrochemical technologies (MET) with natural wastewater treatment biofilters used in constructed wetlands (CW) to form METlands. In order to validate this technology, three lab-scale horizontal subsurface flow (HSSF) biofilters, two hosting electroconductive materials and one gravel biofilter (control) were operated for 525 days to define the best design and operational conditions to maximize the removal of wastewater pollutants. Organic loading rates tested ranged from 2 to 24 g BOD5 m−2 d−1 at hydraulic retention times (HRT) from 4 days to as low as 0.5 day, respectively. The electroconductive biofilter showed the best COD and BOD removal rates per volume of bed, achieving mean values of 213 g COD m−3 d−1 and 119 g BOD m−3 d−1 at the lowest HRT (0.5 d). Ammonia and total nitrogen maximum removal efficiencies at 3.4 days of HRT were 97 and 69%, respectively, in the electroconductive biofilter. Bacterial communities were studied by 16S rDNA Illumina sequencing with the aim of understanding the role of the electrically conductive material in selecting microbial populations. Deltaproteobacteria (a known electroactive taxon) were enriched in the presence of an electrically conductive bed. Geobacter and Geothrix were the dominant genera in the deeper zone of the electrically conductive bed where oxidation of organic matter occurred. The results suggest that the enhancement in biodegradation rate will significantly reduce the area requirements of classical CW.
Water impactWe present for the first time the concept of METland that merges microbial electrochemical technologies (MET) with constructed wetlands. METlands are based on electroconductive biofilters for treating urban wastewater in decentralized systems in a sustainable way with no energy cost. Our strategy was the seed of an innovative European H2020 project devoted to construct full scale applications of METlands (http://www.imetland.eu). |
Introduction
Conventional wastewater treatments require high energy, operation and maintenance costs. In addition, due to population growth and urban expansion, the volume of sewage sludge produced by wastewater treatment is constantly increasing.1 Thus, a different water–energy nexus is required to cope with the future global water demand.Since the discovery of electroactive microorganisms, microbial fuel cells (MFC) have been proposed to play an important role in wastewater treatment for converting waste into clean energy, by oxidizing organic and inorganic matter to generate electrical current.2,3 In these devices, electrons produced by the microbial metabolism are first transferred to an electrode (anode) and then to a second electrode (cathode) via a conductive material containing a resistor.3 In this configuration, the anode acts as a terminal electron acceptor as any other natural acceptor like oxygen, nitrate or Fe(III). The clear advantage of exploiting electro-stimulated communities is that electrodes can boost the microbial metabolism in anaerobic systems that are typically electron acceptor limited. Electroconductive material may represent an inexhaustible source of electron acceptors, hosting the additional advantage of providing a more easily modulated redox potential compared to standard, low-reducing redox species that generally drive these systems.4 The redox potential of the anode depends on the chemistry and bioelectrochemistry around the electrode. Moreover, the electrochemical characteristics of those microbial-assisted devices can be simply controlled by altering their configuration. Thus, they can be operated in different configurations, such as i) short-circuit, no resistors between electrodes;5 ii) MFC, able to harvest energy in the presence of a resistor;6 and iii) microbial electrolysis cell (MEC) by poising a certain potential through a potentiostat or a power source.7,8
A suitable scenario for testing this emergent technology is the constructed wetlands (CW) since they are a good alternative for wastewater treatment in small communities and are used worldwide.9 Low cost operation and maintenance, low energy requirements, low production of sewage sludge (just in primary treatment) and good landscape integration are some of the most attractive advantages of CW compared to conventional treatment systems.10 However, CW treatment is constrained by limitations such as large land requirements (3–10 m2 per PE§ depending on the design)11,12 and clogging by the accumulation of solids.13,14 Recommended surface organic inlet load for HSSF CW is reported as 6.0 g BOD5 m−2 d−1 in order to achieve a value under 30 mg BOD5 L−1 in the effluent and to avoid clogging.15,16 HSSF CW were initially presented as environments that could take advantage of depth-depending redox potential gradients.17,18 Previous reports argued that redox conditions in CW could be controlled by altering the organic loading rate, the hydraulic design and the mode of operation.19 Following this strategy, different groups have integrated MFC elements into lab-scale CW with the purpose of harvesting electricity.20–22 In spite of using wastewater as organic fuel, the power densities reported were as low as 1.84–44.63 mW m−2,23 which is a range typical for sMFC operating in soil or sediments, but still far from 10 W m−2 values obtained using filter press bioelectrochemical reactors.8 This is mainly due to the fact that redox gradients are not broad enough in this kind of environment and in situ implementation of power-harvesting devices is indeed limited.
However, we still believe that CW are a suitable environment for implementing microbial electrochemical systems. Our aim was not to harvest energy but to enhance the rate of pollutant removal by converting the classical inert biofilter into an electroconductive biofilter where its redox state could be tuned or controlled by electrochemical tools. Our results revealed how the integration of METs in wetlands resulted in a powerful hybrid technology, the so-called METland,24 that strongly outperforms the treatment of urban wastewater through the stimulation of different microbial populations.
Experimental
Design and construction of electroconductive biofilters
In this study, four laboratory-scale HSSF biofilters were constructed to determine the best design and operational conditions to maximize wastewater pollutant removal. A control unit used standard siliceous gravel (Ø 6–12 mm) as a biofiltering bed (Fig. 1A). An electroconductive bed configuration (Fig. 1B) was constructed with a single material, acting as a whole electrode. This configuration did not allow the conversion of microbial metabolism into electrical current to be monitored, since the anode and cathode were not differentiated. In order to gain electrochemical information about the process, a three-electrode system was additionally constructed by using a hybrid unit made of inert gravel and a polarized coke bed (Fig. 1C). An additional hybrid unit operating under short-circuit (Fig. 1D) was constructed as control. In these hybrid biofilters, a conductive material was vertically inserted into the gravel. The short-circuit hybrid unit acted as a single electrode without differentiated anode and cathode.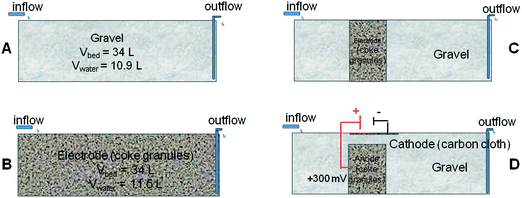 | ||
| Fig. 1 Simplified design of the four systems: A) gravel biofilter (control), B) coke biofilter, C) hybrid biofilter, and D) hybrid polarized biofilter. | ||
The conductive material in the bed was coke granules (Ø 5–10 mm). The dimensions of the biofilters were 0.52 m long, 0.34 m wide and 0.30 m high, and the material layer was 0.20 m deep, with a total bed volume of 0.034 m3 and a water volume of 0.011 m3. Each biofilter had a drainage pipe, located on the flat bottom, for effluent discharge, and the water level was kept below the surface.
The hybrid polarized biofilter hosted a coke anode of 0.006 m3 as shown in Fig. 1. A plate of graphite (3 cm × 3 cm × 0.5 cm, Sofacel) buried into the coke anode acted as an electron collector. The cathode was made of carbon cloth (0.34 m × 0.15 m, Resinas Castro, 420 g m−2). The anode and cathode were connected by a copper wire to a potentiostat unit (Nanoelectra S.L., Spain). A third electrode (Ag/AgCl) buried in the anodic bed acted as reference to polarize the anode at 0.3 V (vs. Ag/AgCl). The anode potential and the current were periodically measured using a digital multimeter (Model 2700, Keithley Instruments, USA). The data were recorded every 10 s on a spreadsheet using ExceLINX_(Keithley) via an interface card (GPIB Interface Boards, Keithley) linked to a personal computer. The performance of the polarized biofilter was evaluated in terms of coulombic efficiency (CE, %) comparing the total electrons harvested by the anode to the electrons possibly generated by the microbial oxidation of the substrate. For continuous flow through the system, we calculate CE based on the COD change and the flow rate, q,25 as follows:
The systems were operated in parallel and fed with real urban wastewater from the municipality of Carrión de los Céspedes (Sevilla, Spain) (2500 inhabitants) under discontinuous flow regime during 525 days (75 weeks). Wastewater was pretreated in an Imhoff tank in order to remove solids and prevent potential clogging of the systems. The feeding from the Imhoff tank was made by programmed pumping, by means of 12 daily periods, simulating the production of wastewater in small populations.26 Several organic loading rates were tested (2.0 ± 1.0, 4.2 ± 0.7, 9.2 ± 2.8, 13.8 ± 9.5 and 24.0 ± 12.7 g BOD5 m−2 d−1 in average) at the following hydraulic retention times (HRT): 4.0, 3.4, 1.7, 0.8 and 0.5 days, respectively.
Physical, chemical and statistical analyses
BOD5, total suspended solids (TSS), total nitrogen (TN), ammonia (NH4) and nitrate (NO3) were analysed weekly; COD was analysed twice a week, following the standard methods.27 Temperature (T), pH, electrical conductivity (EC), dissolved oxygen (DO), and redox potential (ORP) were measured weekly using a handheld multiparameter instrument (YSI 556 MPS). Samples were taken at the inlet and the outlet of the systems and water flow was measured daily. Moreover, hybrid systems were also sampled through sampling tubes buried in the bed, before and after the electroconductive barrier (anode), in order to calculate the coulombic efficiency. Inlet wastewater analyses are shown in Table 1S.† Removal rates were calculated as grams per cubic meter of bed material per day. Removal efficiencies were calculated as percentage.Statistical procedures to evaluate the effect of HRT for every water quality parameter were conducted using the Statgraphics Centurion XVII statistical software package. T-test or Wilcoxon tests were used to determine the differences of every water quality parameter among the effluents, depending on the type of data (95% confidence).
Microbial communities
The Illumina Miseq sequence reads have been deposited in the European Nucleotide Archive (ENA) database under accession Nr. PRJEB10685.
Results and discussion
HSSF CW are biofilter setups that exploit the biofilm-based natural process by means of an inert material like gravel with the purpose of treating urban wastewater. Plants are typically integrated into CW to oxygenate the root zone and to provide aerobic microorganisms a habitat within the anoxic environment.35 Our approach consists in substituting an inert material for an electroconductive material in order to stimulate electroactive microorganisms and consequently biodegradation rates. Due to the oxygen supply role of plants, we did not include vegetal species in our experimental set-up in order to achieve better control of the redox interaction between bacteria and the bed.Urban wastewater treatment by horizontal subsurface flow (HSSF) biofilters: electroconductive versus non-electroconductive biofilters
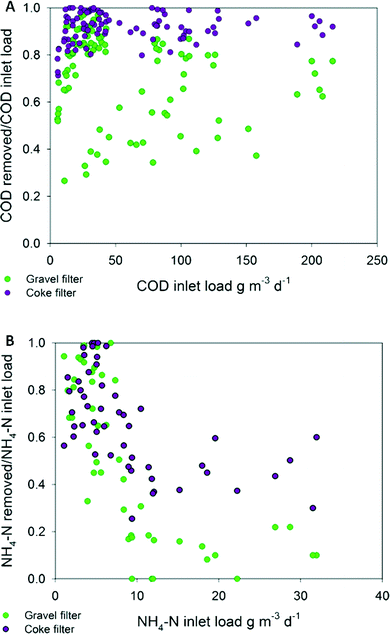
Statistical tests revealed that there were significant differences (p < 0.05) in the effluent's concentration of COD and BOD5 at every HRT (Table 3S†) when the coke and gravel biofilters were compared. The coke biofilter biodegradation rates led to effluents with residual values up to 3-fold lower for COD and 4.5-fold lower for BOD5 (Fig. 3). COD and BOD5 coke biofilter effluent values never exceeded the limits of discharge, which are 125 mg COD L−1 (or >75% removal) and 25 mg BOD5 L−1 (or 70–90% removal) (Dir. 91/271/EEC of 21 May 1991),36 in contrast to the gravel biofilter performance from 3.4 days of HRT onwards, in which the average effluent concentration exceeded 25 mg BOD5 L−1 (Fig. 3). Even at the lowest HRT, the performance of the coke biofilter fulfilled the COD and BOD5 discharge requirements in percentage (91% and 96%, respectively), compared to hardly 73% and 86% for the gravel biofilter (Table 2S†). Caselles-Osorio and García37 reported COD removal efficiencies of 71–85% in intermittent fed HSSF CW experimental systems with a nominal HRT of 3.4 days, which is comparable to removal efficiencies of our control system at the same HRT (83%). The coke biofilter achieved mean BOD5 removal rates as high as 99% at high HRT (3.4 days).
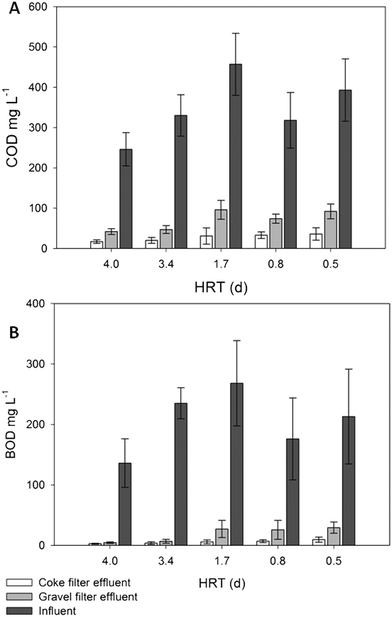 | ||
| Fig. 3 COD (A) and BOD5 (B) influent and effluent average values of the coke and gravel filters. Error bars represent 95% confidence interval. | ||
The BOD5 surface inlet loads applied at 1.7, 0.8 and 0.5 days of HRT (Table 2S†) were 1.5, 2.3 and 4-fold, respectively, the recommended load (6.0 g BOD5 m−2 d−1) and BOD5 average values of the coke biofilter effluent were always under 10 mg L−1 (Fig. 3). Even at very high inlet organic loads, the coke biofilter had a great capacity to remove organic matter, without any evidence of clogging during the long course (525 days) of the experiment. A remarkable conclusion is that only the coke biofilter fulfilled the requirements of the directive for COD and BOD5 at a HRT as low as 0.5 day. In contrast, for a standard gravel biofilter, a HRT as high as 3.5 days was required to fulfill the limits. Moreover, there were no significant TSS differences in the effluents of the two biofilters, and both fulfilled the limit values of discharge (35 mg L−1) (Table 1S†).
Nitrogen removal was also analysed under both electroconductive and inert materials, and a very similar result was found. Statistical analysis revealed significant differences (p < 0.05) among TN and NH4-N effluent concentrations at every HRT. The coke biofilter exhibited the highest removal rate at every HRT (Table 4S†). Interestingly, differences with the gravel biofilter were more noticeable than those found for organic matter removal. In the coke biofilter, the maximum amount of nitrogen was removed at 0.5 day of HRT (TN 11.9 g N m−3 d−1; NH4 11.2 g N m−3 d−1) although the removal efficiency (%) decreased with decreasing HRT. This trend has been reported in other studies.16,38 The coke biofilter showed maximum average removal efficiency values at 3.4 days, 97% of ammonia and 69% of total nitrogen compared to 71% and 51%, respectively, in the gravel biofilter. The minimum values were reached at 0.5 day, 39% of NH4-N and 37% of TN compared to 16% and 19%, respectively, in the gravel biofilter (Table 4S). Fig. 2B shows that the coke biofilter had a trend to maintain higher removal rates than the gravel biofilter. The higher biodegradation rates generated effluents with significantly lower residual TN and NH4-N (Fig. 1S†). The results demonstrate that the coke biofilter removed at least 2-fold the amount of TN and 2.5-fold the amount of NH4 than removed by the gravel biofilter (HRT 0.5 day). Therefore, at a HRT shorter than 4 days, nitrification was higher in the coke biofilter compared to the gravel biofilter. Moreover, at lower HRT, ammonia concentration in the effluent increased, while that of nitrate was decreased (Fig. 1S†). The improvement of the conversion of ammonia to nitrate and nitrogen removal suggests the enhancement of other metabolic pathways in the electroconductive bed.
The electrical current monitored throughout the assay revealed an expected profile, and a stable value around 100 mA was measured (Fig. 4). Interestingly, an increase in the organic loading rate did not result in a clear increase in electrical current, suggesting that the electroactive biofilm was not limited in electron donors. In contrast, the increase in the organic loading rates showed very good correlation with the organic removal rates only in the presence of an electroconductive material so we concluded that some other biodegradation pathways, although not contributing to current production, are definitively being enhanced. As the electron donor is not a limiting factor, other degradation routes must have a major influence on the performance. In that sense, coulombic efficiency (CE) ranged from 37% at a low organic loading rate to 9% at a maximum organic loading rate, which indicates that low organic loading rates enhance the CE. The bacteria can biodegrade part of the COD through fermentation or the use of alternative electron acceptors39 such sulphate or nitrate. This is consistent with previous reports that showed how, under higher organic loading rates, electron flow is channelled towards methanogenesis or sulphate reduction so CE is reduced.39 Methane emissions are common in HSSF CW because these systems present appropriate environmental conditions for methanogens and sulphate-reducing bacteria. These Archaea and Eubacteria require environments with similar redox potentials and use the same types of electron donors (i.e. hydrogen, methanol, and acetic acid).9 Methane emission rates are very variable and they are usually greater at the inlet than the outlet, given that methanogen activity is directly dependent on the organic load.40 Further research about this topic should be carried out to evaluate the contribution of METlands to methane emissions.
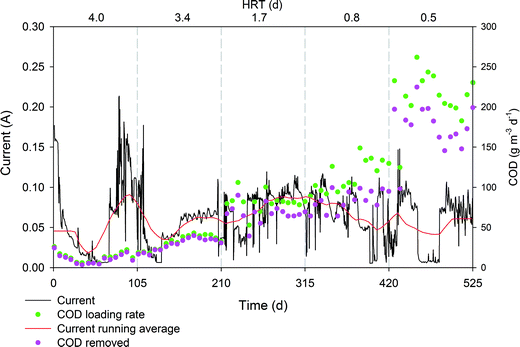 | ||
| Fig. 4 Profile of electrical current, COD loading rate (g m3 d−1) and COD removal rate (g m3 d−1) during long term operation of the hybrid biofilter polarized at 0.3 V (vs. Ag/AgCl). | ||
Together with the hybrid polarized system, a non-polarized hybrid biofilter was also constructed (Fig. 1C) to evaluate the influence of polarization versus the mere effect of the coke. Interestingly, despite polarizing the anode our assays did not reveal significant differences (p > 0.05) in terms of COD and BOD removal among the two hybrid configurations (Fig. 2S†). This fact strongly suggests that the electroconductivity of the material exerts a positive influence on the microbial metabolism regardless of the existence of an electron flow among the different electrodes. Our hybrid biofilter is a single electrode configuration, a simplified design of a short-circuited system that cannot provide current but optimizes the pollutant removal. In that sense, our results are consistent with previous studies that reported how compact short-circuited system provided higher biodegradation performance than MFCs operating at maximum power.41
Redox potential was measured in both the electroconductive and the gravel biofilters. There was a noticeable redox potential gradient with depth and distance from the inlet in the systems which corresponded to COD and BOD. This gradient was greater in the electroconductive biofilter (Fig. 3S†). This gradient suggests the presence of an electron flow from the deep bed to the more oxidized top layer of the coke bed.
In the hybrid systems, the differences between materials were also remarkable. COD removal rates in the electroconductive bed (Table 1) were ca. 5-fold higher than in the gravel bed of the same hybrid device. Regarding nitrogen removal, both hybrid systems removed similar amounts of total nitrogen and ammonia at high and medium HRT (Table 4S†).
| COD levels (mgL−1) | Hybrid biofilter | Hybrid polarized biofilter |
|---|---|---|
| Influent | 231 ± 58 | 231 ± 58 |
| Before the conductive bed | 188 ± 55 | 182 ± 59 |
| After the conductive bed | 89 ± 49 | 78 ± 31 |
| Effluent | 37 ± 20 | 35 ± 14 |
| COD removal | ||
| Removed in the conductive bed (g m−3 d−1) | 50.9 ± 24.8 | 55.5 ± 26.0 |
| Removal efficiency in the conductive bed (%) | 52 ± 18 | 56 ± 14 |
| Removed in gravel before the conductive bed (g m−3 d−1) | 12.8 ± 7.8 | 15.8 ± 13.4 |
| Removed in gravel after the conductive bed (g m−3 d−1) | 10.4 ± 7.0 | 8.1 ± 4.8 |
Microbial communities
The analysis of four microbial communities revealed 696![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 288 raw reads that yielded a total of 689
288 raw reads that yielded a total of 689![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 911 high quality sequences with an average length of 292 bp (Table 5S†). This volume of sequences is around one order of magnitude greater than those of previously reported studies of diversity in bioelectrochemical systems,42 as a result of improved sequencing technologies. Clustering these sequences generated 16
911 high quality sequences with an average length of 292 bp (Table 5S†). This volume of sequences is around one order of magnitude greater than those of previously reported studies of diversity in bioelectrochemical systems,42 as a result of improved sequencing technologies. Clustering these sequences generated 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 582 OTUs evenly distributed between the four samples. 2.33% of the sequence reads were not classified.
582 OTUs evenly distributed between the four samples. 2.33% of the sequence reads were not classified.
The classifiable sequences included members of 48 phyla of which an average of 64% were Proteobacteria, ranging between 52% (anode of the hybrid polarized biofilter) and 74% (gravel biofilter).
Rarefaction curves showed saturation, indicating that a reasonable number of sequence reads per sample were collected to reveal diversity at the sites (Fig. 4S†). Rarefaction curves indicate that predicted diversity was much less in the inlet wastewater than in the rest of the niches (around 70% of the number of identified taxa). Diversity estimators, such as observed OTUs, Chao1 and Shannon-Wienner, were significantly higher for coke granule samples when compared to the gravel samples (Table 5S†). The Good's coverage estimator denoted that the sizes of the libraries were enough to cover almost 100% of the bacterial communities. Shannon diversity indexes (H), which include the information of both richness (the number of species present) and evenness (how the abundance of each species is distributed), were obtained for our system. They were distinctly higher (between 6.27 and 7.38) than those in other studies on electrochemical CW treating urban wastewater (4.36–5.5,43 5.6–6.3 (ref. 44)) and similar to the results of Lu et al.45 (H: 7.33–7.47). These results, together with the high number of taxa found in the samples, indicated a very high diversity.
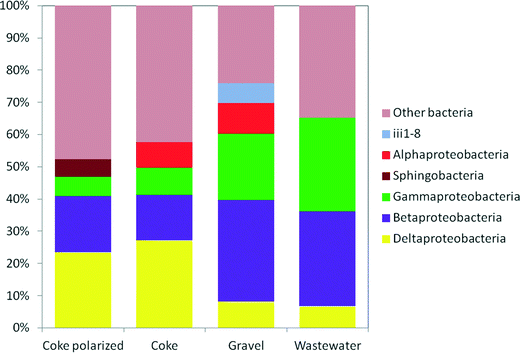
Weighted Fast UniFrac analysis and correspondence analysis (CA) were used to identify the differences in the bacterial community structures based on their phylogenetic lineages. CA showed that the four communities separated distinctly from one another despite the same origin (Fig. 5S†). The CA plot revealed that coke and hybrid polarized biofilters are closely related and that electroactive bacteria (Deltaproteobacteria) had the higher component weight in both systems. Another closely related taxa to these biofilters were the classes Holophagae (with the genus Geothrix, an electroactive bacterium of the phylum Acidobacteria) and Brocadiae (phylum Planctomycetes). The class Brocadiae, involved in Anammox processes, only appeared in the anode of the polarized biofilter (Table 6S†). Alpha, Beta and Gammaproteobacteria had higher component weights in the inlet wastewater and the gravel biofilter.
Some Deltaproteobacteria, like bacteria from the genus Geobacter, are able to transfer electrons to conductive materials.51 Indeed, the largest presence of Geobacter was found in the coke biofilter (2.9%) (Table 7S†). Surprisingly, although at lower levels, it was also found in the inlet wastewater (0.45%) and in the gravel biofilter (0.3%). Some studies have previously reported the presence of Geobacter species in anaerobic digesters suggesting a role in performing direct interspecies electron transfer (DIET)52–55 with a direct impact on methane production. Interestingly, inlet wastewater for our assays was generated in an Imhoff tank, which hosts environmental conditions similar to those found in an anaerobic digester. It seems reasonable to expect the presence of Geobacter associated with other biofilm species in our gravel biofilter. In the Deltaproteobacteria, the dominance of some genera of the family Desulfobulbaceae (Table 7S†) in both the anode of the hybrid polarized biofilter (20.8%) and also in the coke biofilter (16.8%) is remarkable, in contrast to the low presence in the gravel biofilter (1.6%). Moreover, other studies also reported Desulfobulbus species colonizing anodes,44,56–58 and for instance, D. propionicus was previously reported to use the electrode surface as an electron acceptor when pyruvate, lactate, propionate or hydrogen was provided as an electron donor.59 The presence of Desulfobulbus is especially relevant due to its fascinating capacity for generating electrically conductive-microbial filaments.60,61 These microbial filaments transport electrons from the bottom of the sediment, rich in hydrogen sulphide, up to the oxygen-rich sediment that is in contact with the water. Interestingly, this situation is similar to the one found in our METlands where a redox gradient is generated among the bottom and upper layers of the electroconductive bed. Thus, our results have revealed that specific microbial consortia previously related to electrical current production were selected for by our electroconductive biofilters from our inlet wastewater.
On top of that, other electroactive microorganisms like Geothrix, a bacterium of the phylum Acidobacteria,62 were also found in all the systems (Table 7S†), with a significant presence in the anode of the hybrid polarized biofilter (3.2%) and in the coke biofilter (2.2%). Interestingly, Geothrix was almost absent in the inlet wastewater and scarce in the gravel biofilter (0.2%).
A deep analysis into the microbial communities' distribution may help us understand which nitrogen metabolisms are active in our systems. The detection of ammonium oxidizers, like Nitrosomonadaceae, associated with the electroconductive material is remarkable if we consider that this family was absent in both the gravel and the inlet wastewater. Even more interesting was the presence of bacteria from the Brocadiaceae family (1.7%) in the anode of the polarized biofilter. This family of bacteria includes several genera involved in the anaerobic oxidation of ammonia to dinitrogen via Anammox.63
Another nitrogen pathway that could be enhanced by the presence of the electroconductive material is based on direct interspecies electron transfer.64 Focusing on nitrogen removal, it has been reported that Geobacter bacteria can transfer electrons directly to Thiobacillus which in turn may reduce nitrate.65 Interestingly, both microbial genera are colonizing our electroconductive biofilters although further research is required to find out if these redox syntrophic relationships are the ones after nitrogen removal in our systems.
Conclusions
Wastewater treatment problems in small communities are different than those in large cities owing to the scarcity of economical and technical resources. It is necessary to find solutions that have minimum energy cost, simple maintenance and functional robustness. With this aim, the successful integration of microbial electrochemical technologies into well tested treatments, such as constructed wetlands, represents a substantial advance since the new system can be operated at a surface inlet load that is 4-fold higher than the standard systems. Indeed, our lab scale METland design for treating urban wastewater was able to fulfill the requirements of Directive 91/271/EEC and produced water with BOD5 levels as low as 6 mg L−1. Our research suggests that surface area requirements of classical constructed wetlands (CW) can be significantly reduced.Acknowledgements
The research was fully supported by the projects AQUAELECTRA (http://www.aquaelectra.es), SMART WETLAND (http://www.smartwetland.es), and EM4EM funded by the Spanish Ministry of Economy and Competitiveness (MINECO) through both the INNPACTO and the RETOS programmes. The PhD fellowship of Arantxa Aguirre is funded by the Formación de Profesorado Universitario (FPU) programme of the University of Alcalá.References
- M. R. Ghazy, T. Dockhorn and N. Dichtl, Fifteenth Int. Water Technol. Conf. IWTC-15 2011, Alexandria, Egypt, 2011 Search PubMed.
- H. Liu, R. Ramnarayanan and B. E. Logan, Environ. Sci. Technol., 2004, 38, 2281–2285 CrossRef CAS PubMed.
- B. E. Logan, B. Hamelers, R. Rozendal, U. Schröder, J. Keller, S. Freguia, P. Aelterman, W. Verstraete and K. Rabaey, Environ. Sci. Technol., 2006, 40, 5181–5192 CrossRef CAS PubMed.
- A. Kato Marcus, C. I. Torres and B. E. Rittmann, Biotechnol. Bioeng., 2007, 98, 1171–1182 CrossRef PubMed.
- B. Wu, C. Feng, L. Huang, Z. Lv, D. Xie and C. Wei, Bioresour. Technol., 2014, 157, 305–309 CrossRef CAS PubMed.
- K. Rabaey and W. Verstraete, Trends Biotechnol., 2005, 23, 291–298 CrossRef CAS PubMed.
- P. D. Kiely, R. Cusick, D. F. Call, P. A. Selembo, J. M. Regan and B. E. Logan, Bioresour. Technol., 2011, 102, 388–394 CrossRef CAS PubMed.
- Z. Borjas, J. Ortiz, A. Aldaz, J. Feliu and A. Esteve-Núñez, Energies, 2015, 8, 14064–14077 CrossRef CAS.
- J. García, D. P. L. Rousseau, J. Morató, E. Lesage, V. Matamoros and J. M. Bayona, Crit. Rev. Environ. Sci. Technol., 2010, 40, 561–661 CrossRef.
- J. García, J. Vivar, M. Aromir and R. Mujeriego, Water Res., 2003, 37, 2645–2653 CrossRef.
- J. Vymazal and L. Kropfelova, Wastewater Treatment in Constructed Wetlands with Horizontal Sub-Surface Flow, Springer, 2008, vol. 14, p. 579 Search PubMed.
- E. Tilley, C. Lüthi, A. Morel, C. Zurbrügg and R. Schertenleib, Compendium of Sanitation Systems and Technologies, 2008 Search PubMed.
- C. C. Tanner and J. P. Sukias, Water Sci. Technol., 1995, 32, 229–239 CrossRef CAS.
- D. P. L. Rousseau, P. A. Vanrolleghem and N. De Pauw, Ecol. Eng., 2004, 23, 151–163 CrossRef.
- US EPA, Manual Constructed Wetlands Treatment of Municipal Wastewaters, US Environmental Protection Agency, Cincinnati, Ohio, National R., 2000 Search PubMed.
- R. Kadlec and S. Wallace, Treatment wetlands, Taylor & Francis Group, Boca Raton, FL, 2009 Search PubMed.
- C. Corbella, M. Garfí and J. Puigagut, Sci. Total Environ., 2014, 470–471, 754–758 CrossRef CAS PubMed.
- J. Villaseñor, P. Capilla, M. A. Rodrigo, P. Cañizares and F. J. Fernández, Water Res., 2013, 47, 6731–6738 CrossRef PubMed.
- J. L. Faulwetter, V. Gagnon, C. Sundberg, F. Chazarenc, M. D. Burr, J. Brisson, A. K. Camper and O. R. Stein, Ecol. Eng., 2009, 35, 987–1004 CrossRef.
- Z. Fang, H. Song, N. Cang and X. Li, Biosens. Bioelectron., 2015, 68, 135–141 CrossRef CAS PubMed.
- S. Liu, H. Song, S. Wei, F. Yang and X. Li, Bioresour. Technol., 2014, 166, 575–583 CrossRef CAS PubMed.
- Y. Zhao, S. Collum, M. Phelan, T. Goodbody, L. Doherty and Y. Hu, Chem. Eng. J., 2013, 229, 364–370 CrossRef CAS.
- L. Doherty, Y. Zhao, X. Zhao, Y. Hu, X. Hao, L. Xu and R. Liu, Water Res., 2015, 85, 38–45 CrossRef CAS PubMed.
- A. Esteve-Núñez, A. Berná Galiano, A. Reija Maqueda, C. Aragón, A. Aguirre-Sierra, T. Bachetti de Gregoris, R. Esteve-Núñez, B. Barroeta García, J. R. Pidre, J. Fernández Ontivero and J. J. Salas, in MFC4-4th International Microbial Fuel Cell Conference, Cairns, Australia, 2013, pp. 130–131 Search PubMed.
- B. E. B. Logan, Microbial fuel cells, 2008 Search PubMed.
- E. Ortega, Y. Ferrer, J. J. Salas, C. Aragón and Á. Real, Manual para la implantación de sistemas de depuración en pequeñas poblaciones, Ministerio de Medio Ambiente y Medio Rural y Marino, Madrid, 2010 Search PubMed.
- American Public Health Asociation, Standard methods for the examination of water and wastewater, American Public Health Association/American Water Works Association/Water Environmental Federation, Washington DC, 21st edn, 2005 Search PubMed.
- J. G. Caporaso, J. Kuczynski, J. Stombaugh, K. Bittinger, F. D. Bushman, E. K. Costello, N. Fierer, A. G. Peña, J. K. Goodrich, J. I. Gordon, G. A. Huttley, S. T. Kelley, D. Knights, J. E. Koenig, R. E. Ley, C. A. Lozupone, D. McDonald, B. D. Muegge, M. Pirrung, J. Reeder, J. R. Sevinsky, P. J. Turnbaugh, W. A. Walters, J. Widmann, T. Yatsunenko, J. Zaneveld and R. Knight, Nat. Methods, 2010, 7, 335–336 CrossRef CAS PubMed.
- E. Aronesty, Ea-UtilsCommand. Tools Process. Biol. Seq. Data, 2011 Search PubMed.
- R. C. Edgar, Bioinformatics, 2010, 26, 2460–2461 CrossRef CAS PubMed.
- T. Z. DeSantis, P. Hugenholtz, N. Larsen, M. Rojas, E. L. Brodie, K. Keller, T. Huber, D. Dalevi, P. Hu and G. L. Andersen, Appl. Environ. Microbiol., 2006, 72, 5069–5072 CrossRef CAS PubMed.
- M. N. Price, P. S. Dehal and A. P. Arkin, PLoS One, 2010, 5, e9490 Search PubMed.
- N. A. Bokulich, S. Subramanian, J. J. Faith, D. Gevers, J. I. Gordon, R. Knight, D. A. Mills and J. G. Caporaso, Nat. Methods, 2013, 10, 57–59 CrossRef CAS PubMed.
- J. A. Klappenbach, Nucleic Acids Res., 2001, 29, 181–184 CAS.
- H. Brix, Water Sci. Technol., 1994, 29, 71–78 CAS.
- European Union, Directive 91/271/EEC of 21 May 1991, concerning urban waste-water treatment. Official Journal L 135, 30/05/1991 pp. 0040–0052.
- A. Caselles-Osorio and J. García, Sci. Total Environ., 2007, 378, 253–262 CAS.
- C. C. Tanner, J. P. S. Sukias and M. P. Upsdell, J. Environ. Qual., 1998, 27, 448 CrossRef CAS.
- K. Rabaey, P. Clauwaert, P. Aelterman and W. Verstraete, Environ. Sci. Technol., 2005, 39, 8077–8082 CrossRef CAS PubMed.
- S. Teiter and Ü. Mander, Ecol. Eng., 2005, 25, 528–541 CrossRef.
- B. Erable, L. Etcheverry and A. Bergel, Biofouling, 2011, 27, 319–326 CrossRef CAS PubMed.
- J. F. Miceli, P. Parameswaran, D.-W. Kang, R. Krajmalnik-Brown and C. I. Torres, Environ. Sci. Technol., 2012, 120828164231002 CrossRef PubMed.
- C. Corbella, M. Guivernau, M. Viñas and J. Puigagut, Water Res., 2015, 84, 232–242 CrossRef CAS PubMed.
- J. H. Ahn, W. S. Jeong, M. Y. Choi, B. Y. Kim, J. Song and H. Y. Weon, J. Microbiol. Biotechnol., 2014, 24, 1707–1718 CrossRef CAS PubMed.
- L. Lu, D. Xing and Z. J. Ren, Bioresour. Technol., 2015, 195, 115–121 CrossRef CAS PubMed.
- S. Bicciato, M. Pandin, G. Didone and C. Di Bello, Biotechnol. Bioeng., 2003, 81, 594–606 CrossRef CAS PubMed.
- M. J. McInerney, L. Rohlin, H. Mouttaki, U. Kim, R. S. Krupp, L. Rios-Hernandez, J. Sieber, C. G. Struchtemeyer, A. Bhattacharyya, J. W. Campbell and R. P. Gunsalus, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 7600–7605 CrossRef PubMed.
- D. Lovley, Prokaryotes SE - 69, 2013, pp. 287–308 Search PubMed.
- Z. Zhao, Y. Zhang, S. Chen, X. Quan and Q. Yu, Sci. Rep., 2014, 4, 6658 CrossRef CAS PubMed.
- A. Parada, D. M. Needham and J. A. Fuhrman, Environ. Microbiol., 2016, 18, 1403–1414 CrossRef CAS PubMed.
- D. R. Bond, D. E. Holmes, L. M. Tender and D. R. Lovley, Science, 2002, 295, 483–485 CrossRef CAS PubMed.
- M. Morita, N. S. Malvankar, A. E. Franks, Z. M. Summers, L. Giloteaux, A. E. Rotaru, C. Rotaru and D. R. Lovley, mBio, 2011, 2, 5–7 CrossRef PubMed.
- A.-E. Rotaru, P. M. Shrestha, F. Liu, M. Shrestha, D. Shrestha, M. Embree, K. Zengler, C. Wardman, K. P. Nevin and D. R. Lovley, Energy Environ. Sci., 2014, 7, 408–415 CAS.
- P. M. Shrestha, N. S. Malvankar, J. J. Werner, A. E. Franks, A. Elena-Rotaru, M. Shrestha, F. Liu, K. P. Nevin, L. T. Angenent and D. R. Lovley, Bioresour. Technol., 2014, 174, 306–310 CrossRef CAS PubMed.
- Z. Zhao, Y. Zhang, T. L. Woodard, K. P. Nevin and D. R. Lovley, Bioresour. Technol., 2015, 191, 140–145 CrossRef CAS PubMed.
- L. De Schamphelaire, A. Cabezas, M. Marzorati, M. W. Friedrich, N. Boon and W. Verstraete, Appl. Environ. Microbiol., 2010, 76, 2002–2008 CrossRef CAS PubMed.
- Y. Sun, J. Zuo, L. Cui, Q. Deng and Y. Dang, J. Gen. Appl. Microbiol., 2010, 56, 19–29 CrossRef CAS PubMed.
- Z. Wang, T. Lee, B. Lim, C. Choi and J. Park, Biotechnol. Biofuels, 2014, 7, 9 CrossRef PubMed.
- D. E. Holmes, D. R. Bond and D. R. Lovley, Appl. Environ. Microbiol., 2004, 70, 1234–1237 CrossRef CAS PubMed.
- C. Pfeffer, S. Larsen, J. Song, M. Dong, F. Besenbacher, R. L. Meyer, K. U. Kjeldsen, L. Schreiber, Y. A. Gorby, M. Y. El-Naggar, K. M. Leung, A. Schramm, N. Risgaard-Petersen and L. P. Nielsen, Nature, 2012, 491, 218–221 CrossRef CAS PubMed.
- S. Larsen, L. P. Nielsen and A. Schramm, Environ. Microbiol. Rep., 2015, 7, 175–179 CrossRef CAS PubMed.
- D. R. Bond and D. R. Lovley, Appl. Environ. Microbiol., 2005, 71, 2186–2189 CrossRef CAS PubMed.
- B. Kartal, L. van Niftrik, J. T. Keltjens, H. J. M. Op den Camp and M. S. M. Jetten, Adv. Microb. Physiol., 2012, 60, 211–262 CrossRef CAS PubMed.
- A.-E. Rotaru, P. M. Shrestha, F. Liu, B. Markovaite, S. Chen, K. P. Nevin and D. R. Lovley, Appl. Environ. Microbiol., 2014, 80, 4599–4605 CrossRef PubMed.
- S. Kato, K. Hashimoto and K. Watanabe, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 10042–10046 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ew00172f |
| ‡ These authors contributed equally. |
| § PE (population equivalent) is the number expressing the ratio of the sum of the BOD load produced during 24 hours by industrial facilities and services to the individual BOD load in household sewage produced by one person at the same time. For practical calculations, it is assumed that one unit is equal to 60 g of BOD per 24 hours. |
| This journal is © The Royal Society of Chemistry 2016 |