An aerated and fluidized bed membrane bioreactor for effective wastewater treatment with low membrane fouling†
Yaoli
Ye
a,
Nicole
LaBarge
 a,
Hiroyuki
Kashima
a,
Kyoung-Yeol
Kim
a,
Pei-Ying
Hong
b,
Pascal E.
Saikaly
b and
Bruce E.
Logan
*a
a,
Hiroyuki
Kashima
a,
Kyoung-Yeol
Kim
a,
Pei-Ying
Hong
b,
Pascal E.
Saikaly
b and
Bruce E.
Logan
*a
aDepartment of Civil and Environmental Engineering, The Pennsylvania State University, University Park, PA 16802, USA. E-mail: bel3@engr.psu.edu
bBiological and Environmental Sciences and Engineering Division, King Abdullah University of Science and Technology (KAUST), 4700 King Abdullah Boulevard, Thuwal 23955-6900, Saudi Arabia
First published on 23rd September 2016
Abstract
Anaerobic fluidized bed membrane bioreactors (AFMBRs) use granular activated carbon (GAC) particles suspended by recirculation to effectively treat low strength wastewaters (∼100–200 mg L−1, chemical oxygen demand, COD), but the effluent can contain dissolved methane. An aerobic fluidized bed membrane bioreactor (AOFMBR) was developed to avoid methane production and the need for wastewater recirculation by using rising air bubbles to suspend GAC particles. The performance of the AOFMBR was compared to an AFMBR and a conventional aerobic membrane bioreactor (AeMBR) for domestic wastewater treatment over 130 d at ambient temperatures (fixed hydraulic retention time of 1.3 h). The effluent of the AOFMBR had a COD of 20 ± 8 mg L−1, and a turbidity of <0.2 NTU, for low-COD influent (153 ± 19 and 214 ± 27 mg L−1), similar to the AeMBR and AFMBR. For the high-COD influent (299 ± 24 mg L−1), higher effluent CODs were obtained for the AeMBR (38 ± 9 mg L−1) and AFMBR (51 ± 11 mg L−1) than the AOFMBR (26 ± 6 mg L−1). Transmembrane pressure of the AOFMBR increased at 0.04 kPa d−1, which was 20% less than the AeMBR and 57% less than the AFMBR, at the low influent COD. Scanning electron microscopy (SEM) analysis indicated a more uniform biofilm on the membrane in AOFMBR than that from the AeMBR biofilm, and no evidence of membrane damage. High similarity was found between communities in the suspended sludge in the AOFMBR and AeMBR (square-root transformed Bray–Curtis similarity, SRBCS, 0.69). Communities on the GAC and suspended sludge were dissimilar in the AOFMBR (SRBCS, 0.52), but clustered in the AFMBR (SRBCS, 0.63).
Water impactA new type of membrane reactor was developed that combined aerobic conditions and a fluidized bed of granular activated carbon (GAC) to better control membrane fouling and enable effective wastewater treatment without the generation of methane gas. Aerobic conditions led to different microbial communities on the GAC and in suspension compared to similar communities in the completely anaerobic reactor. |
1. Introduction
Aerated membrane bioreactors (AeMBRs) are effective alternatives to conventional processes for wastewater treatment because of the combination of good biochemical oxygen demand (BOD) removal and the lack of a need for a secondary clarifier. Other advantages of AeMBRs include the ability to obtain a high mixed liquor suspended solids (MLSS) concentration in the reactor,1 stability of performance during fluctuations in flow and organic loading, low excess sludge production, and relatively short hydraulic retention times (HRTs).2,3 However, a major disadvantage of AeMBRs and other membrane bioreactors (MBRs) is the need for frequent membrane chemical cleaning to avoid excessive membrane fouling.4 The energy demands of all aeration systems, including AeMBRs and activated sludge, are also high compared to those needed for anaerobic treatment techniques.5Anaerobic membrane bioreactors (AnMBRs) are being developed as alternatives to activated sludge and aerated membrane bioreactors in order to reduce energy demands needed for wastewater treatment6 because AnMBRs do not require aeration, and to reduce treatment plant operating costs as anaerobic processes can produce less sludge than aerobic systems. AnMBRs have been tested with many types of wastewaters, including municipal, synthetic, food processing, and industrial, at both laboratory and pilot scales, and have produced good effluent quality.6,7 However, membrane fouling is also challenging for AnMBR operation. Various strategies have been developed to reduce fouling, such as biogas recirculation and sparging,8 addition of granular or powdered activated carbon (PAC) as an absorbent9 in a submerged membrane operation, ultrasonic irradiation,10 and high cross flow velocity11 for the external cross-flow operation.
A new approach to reduce membrane fouling for low-strength wastewaters was recently developed based on using fluidized granular activated carbon (GAC), in a process called an anaerobic fluidized bed membrane bioreactor (AFMBR). The AFMBR has primarily been used as the secondary treatment reactor to treat the effluent from several different types of reactors. In tests using effluent from an anaerobic fluidized bed bioreactor (AFBR) treating synthetic wastewater, the AFMBR achieved 87% removal of the chemical oxygen demand (COD) (influent of 59 mg COD L−1) and nearly 100% solids removal, at an HRT of 2–3 h. Membrane fouling was well controlled as the reactor was operated for 120 days, and required only two chemical cleanings. The energy consumption of the AFMBR was estimated to be only 0.058 kW h m−3.12 An AFMBR was also used as a secondary treatment process to treat effluent from a microbial fuel cell (MFC) treating domestic wastewater. At an HRT of only 1 h, the AFMBR removed 85% of the COD and 99.6% the TSS, with an estimated energy demand of 0.0186 kW h m−3.13 An AFMBR treating the effluent from an anaerobic baffled reactor (ABR), showed 87% COD removal using a complex synthetic wastewater at an HRT of ∼1 h, with an energy demand of 0.0087 kW h m−3.14 A single AFMBR was compared to staged anaerobic fluidized membrane bioreactors (SAF-MBR) for treating synthetic wastewater (∼200 mg COD L−1), with no significant differences found between the processes in terms of COD removal efficiency, transmembrane pressure (TMP), bulk liquid suspended solids, extracellular polymer substances (EPS) production, and soluble microbial products (SMP).15
One of the main disadvantages of AnMBRs or AFMBRs is that the effluent can contain high concentrations of dissolved methane which must be removed prior to discharge to avoid the release of this greenhouse gas to the atmosphere.16,17 In this study, an aerobic fluidized bed membrane bioreactor (AOFMBR), containing 92 g L−1 fluidized GAC particles as scouring media, was examined to simultaneously avoid production of dissolved methane, as well as eliminate the need for water (AFMBR) or biogas recirculation (AnMBR) used in anaerobic membrane reactors. The performance of the AOFMBR was compared in side-by-side tests with two other processes as controls: an AFMBR and an AeMBR. Domestic wastewater was used at three different COD concentrations (∼150, 200, and 300 mg L−1) to study the impact of organic loading on COD removal and membrane fouling. It was hypothesized that AOFMBR could achieve better organics removal and have less membrane fouling, as well as avoid generation of dissolve methane due to the aerobic conditions compared to the AFMBR. Treatment was evaluated in terms of COD and soluble COD (SCOD) removal and effluent turbidity, and TMP was monitored to assess membrane fouling. Scanning electron microscopy (SEM) was conducted to examine the morphology of biofilms on the membrane and membrane integrity. Analysis of microbial communities by 16S rRNA gene sequencing was done by sampling the solutions, and when present, the GAC, in the different reactors.
2. Materials and methods
2.1. Reactor design
All three reactors (AFMBR, AOFMBR, and AeMBR) were constructed from polyvinyl chloride (PVC) tubes (30 cm long and 1.6 cm in diameter) with fittings and connectors as previously described.13 Each reactor had a volume of 65 mL, with slightly different configurations for aeration versus recirculation (Fig. S1†). A thick butyl rubber stopper (20 mm diameter, Chemglass Inc., Vineland, NJ) was placed on the top of the AFMBR tube to keep it sealed. Biogas produced by the AFMBR was collected for analysis in a gas bag to the top of the reactor using a needle to pierce the rubber stopper. Each reactor contained a membrane module made by bundling eight polyvinylidene fluoride (PVDF) hollow fiber membranes (24 cm long, 2.0 mm in outer diameter, 0.8 mm in inner diameter, 0.1 μm pore size, Kolon Inc., South Korea) together for a total exposed membrane area of 0.0048 m2 per reactor. Epoxy was applied as the sealant. The module was placed in the middle of the reactor body, with the effluent pulled through the membrane by suction generated using a peristaltic pump (model no. 7523-90, Masterflex, Vernon Hills, IL). GAC (DARCO MRX, 10 × 30 mesh, Norit Activated Carbon) was rinsed with deionized water several times, and added into the AFMBR (10 g wet weight, 153 g GAC L−1) and AOFMBR (6 g wet weight, 92 g GAC L−1). High concentrations of GAC were used to control membrane fouling by scouring in both the AFMBR and AOFMBR reactors, at concentrations similar to those used in previous AFMBR studies.13,18 Less GAC was added into AOFMBR in order to keep it better fluidized by rising air bubbles. AeMBR and AOFMBR had the same reactor configuration except no GAC was added into AeMBR. Mechanical scouring of GAC (AFMBR and AOFMBR) or shear generated by air bubbles (AeMBR) were the only strategies to control membrane fouling during the study. There was no chemical cleaning of the membranes.2.2. Reactor operation
All three reactors were operated at an overall HRT of 1.3 h (11.6 L m−2-membrane-h), with pumps operating for 10 min on at 0.93 mL min−1, and 1 min off for relaxation of the membranes. Fluid was recirculated by pumping (model no. 7523-90, Masterflex, Vernon Hills, IL) at a flowrate of 250 ± 30 mL min−1 (upflow velocity of 2.4 cm s−1) to fluidize the GAC in the AFMBR. An air flow of 240 ± 20 mL min−1 (3.0 m3 m−2-membrane surface-h) was used for both the AOFMBR and AeMBR, by placing a gas diffusor (gas dispersion tubes, Medium Frit, Chemglass, US) at the bottom of the reactor (Fig. S1†). The effluent tubing was cleaned with hydrochloric acid two times (day 41 and 89) in response to spikes in effluent turbidity, to remove accumulated biomass growing in the tubing.In order to acclimate the microorganisms for growth on the GAC, the AFMBR and AOFMBR were inoculated using diluted municipal wastewater (primary clarifier of the Pennsylvania State University Wastewater Treatment Plant) with a COD of 150 mg L−1, for one month, using the operation mode described above. The AeMBR was fed with the same diluted wastewater for one month. To begin the experiments, the membrane modules were replaced by new ones (designated day 1).
In order to study the effect of wastewater strength on membrane fouling and effluent quality, the study conditions were separated into 4 phases: phase 1 (1–45 days), influent COD of 153 mg L−1; phase 2 (45–73 days), influent COD of 214 mg L−1; phase 3 (73–103 days), influent COD of 299 mg L−1; phase 4 (103–131 days), influent COD of 329 mg L−1, and using a new membrane module. Operational details are summarized in Table 1. The replacement of the membrane module for phase 4 was needed due to the TMP drop in the AFMBR and AeMBR, which might have been caused by the failure of the membrane at some time in phase 3. The wastewater strength was controlled by dilution of domestic wastewater using distilled water to obtain the targeted COD. Consistent pH and solution conductivity were obtained by adding sodium bicarbonate and sodium chloride as needed to each diluted wastewater sample (pH of ∼7.6 and conductivity of 1.2 mS cm−1). Effluent samples were taken from the effluent tubing every two days. All reactors were operated at ambient temperature (22 ± 10 °C).
| Phase | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| COD (mg L−1) | 153 ± 19 | 214 ± 27 | 299 ± 24 | 329 ± 37 |
| SCOD (mg L−1) | 87 ± 18 | 115 ± 19 | 185 ± 26 | 179 ± 28 |
| pH | 7.7 ± 0.1 | 7.7 ± 0.1 | 7.5 ± 0.3 | 7.7 ± 0.2 |
| Conductivity (mS cm−1) | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.3 ± 0.1 | 1.2 ± 0.1 |
| Membrane | New | Continued | Continued | New |
2.3. Measurement and analysis
COD was measured using a DR3900 Spectrophotometer (HACH, Company, Loveland, CO). Samples for SCOD analysis were filtered using 0.45 μm pore size syringe filters (PVDF, 25 mm size, Restek Corporation). Differences in effluent CODs and SCODs between the reactors were assessed using the Student's t-test. The difference in results was considered to be significant here when the P value was less than 0.03. Conductivity and pH were measured using probes and meters (Seven-Multi, Mettler-Toledo International Inc.). Biogas production rates were calculated from the change in biogas composition (measured using gas chromatographs; SRI Instruments) and volume of gas in the collection bag. Turbidity was measured (2100P, HACH Company, Loveland, CO) as an indicator of solids removal. Pressure in the effluent tubing was monitored as the TMP of the membrane module using a pressure transducer (TDH 31, Transducer Direct, US). Suction pressure was reported as a positive value. TMP and turbidity data were collected starting on day 7.Membranes from the reactors were examined with SEM at the end of phase 3 to evaluate biofilm formation due to the three different reactor operational conditions. Small pieces of membrane were cut from the middle of the fiber, and prepared by: fixation in 2.5% glutaraldehyde in a 0.1 M phosphate buffer solution (PBS) at a pH of 7.2 for 30 minutes; rinsing with PBS for 3 × 5 min; successive dehydration using ethanol solutions of 5%, 50%, 70%, 85%, 95%, 3 × 100%, each for 5 min; critical point drying; sample mounting on aluminum stubs with conductive tabs; and coating with 10 nm Au/Pd. SEM images were viewed at 125× and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× magnifications.
000× magnifications.
The microbial communities were analyzed using genomic DNA extracted from the suspended biomass and biomass on the GAC (0.25 g, if present) in the AOFMBR, AFMBR, and AeMBR at the end of phase 4. For suspended biomass samples, liquid (13 mL) from the reactor was centrifuged at 4500 × g (Eppendorf 5804) for 1 h and the supernatant was decanted. For the AOFMBR and AeMBR suspended biomass analysis, 0.25 g of pellet was used for DNA extraction, but less was used for the AFMBR due to less solids collected. DNA was extracted from suspended biomass samples and GAC following the power soil DNA isolation kit protocol (Mo Bio Laboratories, Inc) with modifications: 0.1 um diameter glass beads were used instead of garnet beads; samples were centrifuged for 1 min instead of 0.5 min; and the incubation time was increased for 10 min instead of 5 min. The 16S rRNA genes in the extracted DNA samples were amplified by polymerase chain reaction (PCR) according to a previous study.19 Briefly, thermal cycling was conducted with the barcoded forward primer of 515F (5′-Illumina overhang -GTGYCAGCMGCCGCGGTA-3′) and reverse primer 805R (5′-Illumina overhang-GACTACHVGGGTATCTAATCC-3′), followed by the purification of amplicons. Equimolar 16S rRNA gene amplicons were mixed and submitted for high-throughput amplicon sequencing on an Illumina MiSeq platform (Illumina Inc, San Diego, CA, USA) in the KAUST Genomics Core Lab. The DNA sequences were processed for its quality and analyzed by the same approach as specified in previous study.20 Briefly, the relative abundances of various microbial genera and unclassified groups were square-root transformed and calculated for Bray-Curtis similarities (SRBCS) and metric multi-dimensional scaling (mMDS). Microbial groups that exhibited more than 0.95 Pearson's correlation to the spatial distribution of samples were overlaid onto the mMDS plot, and represented as vectors accounting for the spatial positions of samples. The microbial relative abundance was plotted in phylum level, with the predominant genus (>1%) shown separated. All high-throughput sequencing files were deposited in the short read archive (SRA) of the European Nucleotide Archive (ENA) under study accession number PRJEB13756.
3. Results
3.1. Effluent quality and COD removal
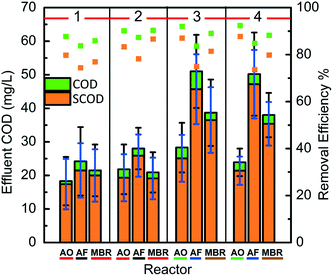
The AOFMBR had the lowest effluent COD and SCOD among all three reactors on average, for tests with the influent COD of either 153 ± 19 mg L−1 (phase 1) or 214 ± 27 mg L−1 (phase 2). In phase 1, the average effluent COD from the AOFMBR was 18 ± 7 mg L−1, and the SCOD was 17 ± 8 mg L−1 (Fig. 1). These corresponded to removal efficiencies of 88 ± 4% for COD, and 80 ± 9% for SCOD. COD removal efficiencies averaged 84 ± 6% for the AFMBR, and 86 ± 5% for the AeMBR. Good treatment was therefore also obtained by AFMBR and AeMBR, as the effluent CODs and SCODs were all <30 mg L−1.When the organic loading rate was increased by 40% using an influent COD of 214 ± 27 mg L−1 (phase 2), effluent COD concentrations from the AOFMBR (COD, 22 ± 7 mg L−1; SCOD, 19 ± 7 mg L−1) were not significantly different from those in phase 1 (t-test, P = 0.13), resulting in an improved removal efficiency of 90 ± 3% for COD and 82 ± 7% for SCOD (Fig. 1). Increased COD removals were also obtained for the AFMBR (86 ± 4%) and AeMBR (90 ± 3%). Thus, for phase 1 and 2 tests, the effluent from all reactors had good effluent qualities based on the effluent CODs.
When the influent COD was increased by another 40% to 299 ± 24 mg L−1 (phase 3), the AOFMBR had better treatment performance than the other two reactors. The AFOMBR effluent COD increased slightly to 28 ± 7 mg L−1 (from 22 ± 7 mg L−1) (Fig. 1) in phase 3, resulting in average removal efficiencies of 91 ± 2% for COD and 86 ± 3% for SCOD. The effluent CODs were much higher for the other two reactors, with 39 ± 10 mg L−1 (87 ± 3% removal) for the AeMBR, and 51 ± 11 mg L−1 (83 ± 3% removal) for the AFMBR. The effluent CODs of the AOFMBR were significantly different from those of the AFMBR (t-test, P < 0.001) and AeMBR (t-test, P = 0.002). In addition, the effluent CODs of all three reactors in phase 3 were significantly different from those in phase 2 (t-test, AFMBR, P < 0.001, and AOFMBR, P = 0.001, and AeMBR, P < 0.001). The AOFMBR had slightly improved COD and SCOD removals compared with phase 1 and 2, while the percent COD removal decreased for the AFMBR and AeMBR.
Due to an unusual drop in TMP at the end of phase 3, possibly due to a failure of the membrane or the epoxy seal, new membrane modules were installed in all reactors on day 103 (start of phase 4). The influent COD of 329 ± 37 mg L−1 was not significantly different than that in phase 3 (t-test, P = 0.04). The effluent CODs from each reactor with the new membrane module were not significantly different from those obtained in the previous phase 3 (t-test, AFMBR, P = 0.43, and AOFMBR, P = 0.04, and AeMBR, P = 0.42) (Fig. 1), indicating that the membrane condition (after 103 d of operation for phase 1–3 or a new membrane) had little impact on organics removal.
3.2. Effluent turbidity
During the 131 d operation period, the AOFMBR effluent consistently had a very low turbidity of 0.2 ± 0.1 NTU (Fig. 2). The AeMBR had a comparable effluent turbidity (0.2 ± 0.1 NTU), but the AFMBR effluent turbidity was higher and more variable (0.9 ± 1.3 NTU). There were several spikes in the effluent turbidity of the AFMBR, with values as high as 6.0 NTU (day 23) and 8.2 NTU (day 27). However, these spikes in turbidity were likely a result of biomass growth and detachment from the effluent tubing. When the tubing of the AFMBR was cleaned, the turbidity immediately decreased, for example from 1.4 to 0.2 NTU (day 41), and from 1.5 to 0.3 NTU (day 89). After about another half month of operation the effluent turbidity of AFMBR increased to 1.2 (day 59) and 1.1 (day 99) NTU, but no such effluent turbidity increases were measured for effluent samples from the AOFMBR and AeMBR. Even when the very high turbidity spikes (>2 NTU) were removed from the analysis, the average turbidity of AFMBR was 0.6 ± 0.4 NTU (Fig. 2), which was 300% as high as the other two aerobic reactors. This suggests that effluent quality in terms of turbidity was better in the aerated reactors (AOFMBR and AeMBR) than the anaerobic reactor (AFMBR).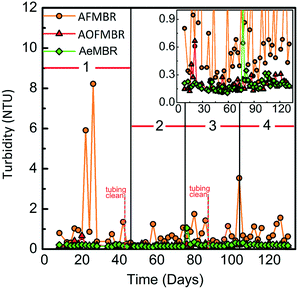 | ||
| Fig. 2 Effluent turbidity over time measured for the three different types of membrane bioreactors. The inset shows the low turbidity range. | ||
3.3. Transmembrane pressures
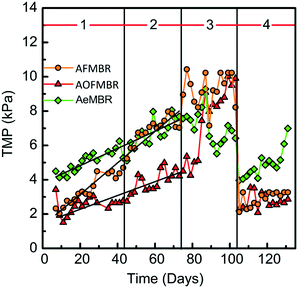
In the first two phases (lower influent COD concentrations), the TMP of the three reactors all gradually increased over time, which indicated the membrane flux of 11.6 L m−2 h−1 was below the critical flux, above which quick cake layer deposition occurs.6 The AOFMBR had the slowest increase rate in TMP (0.04 kPa d−1, obtained by linear fitting) among the three reactors (Fig. 3), increasing from 1.9 kPa (day 7) to 4.2 kPa (day 73). The AFMBR had a starting TMP similar to that of the AOFMBR, but the TMP increased at a rate of 0.09 kPa d−1, reaching 7.0 kPa at the end of phase 2 (day 73), which was 67% larger than that of the AOFMBR. The AeMBR had a much higher initial TMP of 4.1 kPa, reaching 7.7 kPa by the end of phase 2, which was the highest of the three reactors. In general, the increase in TMP showed good agreement with a linear fit of the data in phases 1 and 2 (Fig. 3), suggesting there was no appreciable change in membrane fouling due to the increased COD in these two phases. The relative proportion of the increased TMP rate, based on the slopes of the three lines in Fig. 3, were 1.25 for the AeMBR and 2.3 for the AFMBR relative to the slope for the AOFMBR.In phase 3, there were large increases in TMP for both the AOFMBR and AFMBR but not the AeMBR. The AOFMBR had a particularly sudden increase in the TMP, reaching 9.9 kPa by the end of phase 3, similar to that of the AFMBR (10.2 kPa). The AeMBR exhibited much different behavior, and the TMP decreased over phase 3, which might be a result of membrane failure or failure of the membrane fittings. After replacing all membrane modules for continued testing phase 4, the TMP showed a trend similar to that in phase 1, with slow increases in the TMP. The AeMBR had a higher initial TMP in phase 4 than the other two reactors, consistent with results from phase 1.
3.4. SEM imaging
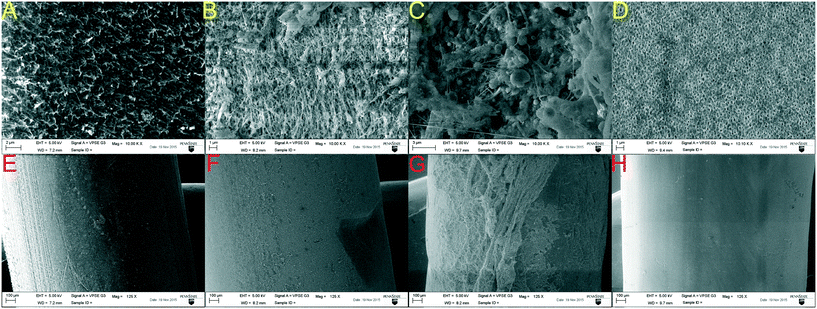
Biofilm growth was observed on the AOFMBR (Fig. 4B) and AeMBR membranes (Fig. 4C), based on comparisons with abiotic membranes (Fig. 4D). The AOFMBR biofilm was relatively uniform (Fig. 4F), but it appeared to have a coarser morphology (Fig. 4B) than those on the AFMBR (Fig. 4A), which had a relatively smooth and uniform morphology (Fig. 4A and E). However, no objects with sizes similar to those of bacteria were found on the AFMBR membrane surface (Fig. 4A). Without GAC addition, the biofilm on the AeMBR membrane surface was the most heterogeneous (Fig. 4C) and non-uniform (Fig. 4G) of the different membranes examined. No sign of membrane surface damage was observed on all the membrane imaged.3.5. Microbial community analysis
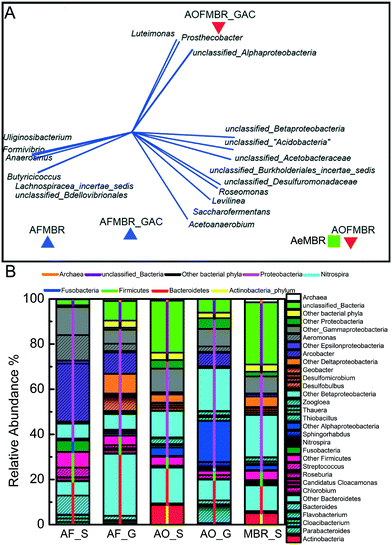
The microbial communities in the reactors formed three distinct groups based on clusters observed in mMDS plots (Fig. 5A). The microbial communities of the suspended solids in the two aerobic AOFMBR and AeMBR reactors were relatively more similar to each other (SRBCS, 0.69) than the other samples from the AFMBR or the GAC in the AOFMBR (Fig. 5A). The suspended solids microbial communities in the AFMBR were different from those in the two reactors, but were similar to communities on the GAC in that reactor (SRBCS, 0.63). The microbes on the GAC in the AOFMBR were distinct from all other samples.The dominant phyla in all the samples were Proteobacteria (42% to 66%), Bacteroidetes (12% to 31%), Firmicutes (4% to 13%), while Actinobacteria was found to be abundant only in the suspended solids in the aerobic reactors (9% in AOFMBR and 5% in AeMBR) (Fig. 5B). Fusobacteria was found to be only abundant in the suspended solids in AFMBR (5%). At the genera level, there was no large predominance of any single genera in the suspended solids in AOFMBR and AeMBR. However, in the AOFMBR, Arcobacter (7%) and Flavobacterium (10%) were the predominant genus on the GAC (Fig. S3†). There was also approximately five times higher relative abundance of Nitrospira (1.2%) on the GAC in the AOFMBR compared to the liquid samples in AOFMBR and AeMBR. Similarly, the GAC of AOFMBR had Nitrosomonas present at relative abundance of 0.03% while Nitrosomonas was present at a lower relative abundance in the suspended solids from the AOFMBR (0.006%) and AeMBR (0.003%). In contrast to the nitrifying populations, methane-oxidizing bacteria (e.g. Methylomonas, Methylosarcina and Methylococcus) were present at 145-fold higher relative abundance in the suspended solids of AOFMBR (average 0.03%) compared to that detected on the GAC in the AOFMBR. The abundance of methane-oxidizing bacteria in the suspended solids of AOFMBR was higher than that detected in the AeMBR (0.002%) and much higher than that in the AFMBR (0.001%).
The same dominant genera, Arcobacter, was found in the suspended solids (31%) and GAC (13%) from the AFMBR, consistent with their close clustering in the mMDS plots. However, some other genera were present at different relative abundances on the GAC compared to those in the solution. For example, Geobacter was present at up to 7.8% relative abundance on GAC, but only <0.2% in the suspended solids, in the AFMBR. Methanogenic archaeal sequences were retrieved in higher relative abundance on the GAC from the AFMBR than the suspended solids. For example, Methanothrix was present in 0.2% on the GAC compared to 0.02% in the suspended solids in AFMBR, while Methanospirillum had a 4-fold higher relative abundance on the GAC (0.04%) than in the suspended solids (0.01%).
3.6. Energy production and consumption
The production of methane gas in phases 3 and 4 averaged 6.2 ± 1.2 L m−3-wastewater treated. No methane gas was collected and analyzed in the first two phases of reactor operation. Dissolved methane was detected in the AFMBR effluent but not in the effluents of the aerated reactors (AOFMBR and AeMBR). The methane generated by the AFMBR could be used to produce 0.02 kW h m−3 of electricity [assuming a 33% conversion efficiency of methane to electricity13]. However, the amount of methane produced here would not be sufficient to provide the energy needed to strip the dissolved methane out of the effluent, which was estimated to be 0.05 kW h m−3.21 On this basis of energy recovery versus that needed for methane gas stripping, avoiding methane generation could be more economically favorable than harvesting methane with potential energy cost for air stripping.The main advantage of using an AOFMBR compared to the AFMBR was avoiding the production of methane gas. However, it would also be desirable to reduce the energy for suspension of the GAC by aeration to be less than that needed for recirculation in the AFMBR. Based on the energy used here for aeration, however, using the air to replace recirculation did not provide a favorable energy balance (Table 2). The energy consumption in AOFMBR, as well as AeMBR (data not shown in Table 2), was still four times as high as that of AFMBR (energy calculation details are in ESI†). The main part of energy consumption in the AOFMBR was aeration, while the majority of energy needed for the AFMBR would be that used for methane gas stripping. The effluent pumping energy to drive the suction pressure of the membrane module was estimated to be only 0.6% of total energy in AOFMBR, and 4.3% in AFMBR. Even though the AOFMBR had an advantage compared to the AFMBR of less membrane fouling under low influent COD conditions, the reduced energy needed for the AOFMBR due to the lower TMP would not be sufficient to make it less costly to operate than the AFMBR. Although sludge production was not monitored in this study, and no sludge was removed from the reactor over the 131 day study, it will be necessary to consider the cost for sludge treatment in this aerobic system compared to the anaerobic AFMBR.
| Energy estimation | AFMBR (kW h m−3) | AOFMBR (kW h m−3) | AeMBR (kW h m−3) |
|---|---|---|---|
| Stripping energy from ref. 21. NA: not applicable. | |||
| Recirculation pumping | 0.019 | NA | NA |
| Air blower | NA | 0.24 | 0.24 |
| Influent pumping | 0.0014 | 0.0014 | 0.0014 |
| Effluent pumping | 0.0026 | 0.0013 | 0.0022 |
| Methane stripping | 0.05 | NA | NA |
| Energy generation from methane | −0.02 | NA | NA |
| Total | 0.06 | 0.24 | 0.24 |
4. Discussion
4.1. Enhanced effluent quality and membrane fouling control
The use of rising bubbles in the AOFMBR to suspend the GAC, rather than recirculation in the AFMBR, resulted in improved treatment, with a 49% lower effluent COD (average of 26 ± 6 mg L−1) for the AOFMBR compared to the AFMBR (51 ± 11 mg L−1). The use of GAC in the aerated AOFMBR also improved removal compared to the AeMBR, with a 32% lower effluent for the AOFMBR than the AeMBR (38 ± 9 mg L−1), at the highest influent CODs of 299 mg L−1 (phase 3) and 329 mg L−1 (phase 4) at a HRT of 1.3 h. This improvement in performance for the AOFMBR compared to the AeMBR was due to the presence of the GAC, which may have reduced the COD levels by providing a high surfaced area for growth of microorganism and adsorption of the organic matter. The COD removals of the AFMBR (84–87%) in this study were within the large range of those previously reported of 63%22 to 95%15 using various wastewaters and HRTs. However, COD removals in most of these studies have ranged from 80% to 90%.12–14 AeMBRs treating domestic wastewater (low strength) generally are in the range of 80% to 95% COD removal,12,23 which is consistent with that observed here (86–89%) even though the AeMBR was not chemically cleaned during this study. The AOFMBR consistently produced an effluent with a low turbidity (0.2 ± 0.1 NTU) that was on average 78% lower than that of the more highly variable effluent turbidity of the AFMBR (0.9 ± 1.3 NTU). A low effluent turbidity was also obtained for the AeMBR (0.2 ± 0.1 NTU), consistent with a previous study.24Membrane fouling was better controlled for the AOFMBR compared to the AFMBR and AeMBR, when the influent COD was lower than 200 mg L−1, as the TMP increase rate was only 45% of that in AFMBR and 80% of that in AeMBR. Biofilm was observed on the membrane of the AOFMBR, while no bacteria-size particles were found on the membrane of AFMBR, suggesting accumulated material was likely biomass debris, SMP or precipitated inorganics. The different surface morphologies suggest that the aerobic conditions or the way the GAC scoured the membranes when air bubbles were present was also important for achieving a reduction in the rate of membrane fouling. The effectiveness of GAC for scouring and minimizing membrane fouling was supported by SEM images, as the biofilm on the AOFMBR membrane appeared to be relatively uniform compared to the more heterogeneous biofilm on the AeMBR membrane. When a thick biofilm or mass of particulate organic matter forms on a membrane, it is referred to as the cake layer, and it usually is the major part of the membrane resistance.25 Therefore, the reduction of membrane fouling in the AOFMBR can be explained primarily by the effective reduction of the thickness of a cake layer by fluidizing GAC. One concern in using both air bubbles and high concentration of GAC (92 g L−1) in the AOFMBR was the potential for damage of the membrane. However, there was no evidence of loss of membrane integrity in the system due to the high concentration of GAC and presence of air bubbles based on either visual observations or reactor performance.
While GAC has previously been used in other types of aerated membrane reactors, the concentration of GAC used here in AOFMBR (92 g L−1) was similar to that used in anaerobic reactor AFMBR studies (95–342 g L−1),12–14,18,26 but much higher than those used in aerated membrane reactors in many previous studies (up to 2 g L−1). Thus, the use of a high concentration of GAC, which is a good adsorbent of organic matter, likely aided in reducing membrane fouling as biopolymers and organics in the AOFMBR or AFMBR could be adsorbed by the GAC rather than deposited on the membrane surfaces. In a previous study, when only a relatively small amount (2 g L−1) of powdered activated carbon (PAC) was added into an AeMBR, improved treatment was obtained for a distillery wastewater.27 Adding 0.75 g L−1 or 1.5 g L−1 of PAC into an AeMBR was also previously shown to reduce membrane fouling.24 GAC addition of 0.5 to 2 g L−1 was also found in another study to minimize sudden increases in membrane resistance, and organic removal was improved.28 It was concluded in all these other studies that the reduction in fouling was mainly due to adsorption of foulants onto the activated carbon, although scouring may have also been important. In the AOFMBR conditions examined here, the substantially higher GAC concentration compared to these previous studies made it possible that organic matter adsorption to the GAC was a factor, in addition to membrane scouring, in minimizing membrane fouling. The GAC in both the AFMBR (10 g) and the AOFMBR (6 g) was not replaced during this study (more than 200 days, including the acclimation and test periods), and there was no sign of reduced performance at the end of phase 4 due to the age of the carbon. While carbon replacement might be needed for operation over longer periods of time, the rate that carbon might need to be replaced cannot be estimated based on the results of this current study.
The TMP of 10.2 kPa that developed in the AFMBR is within the range of 5 kPa to 20 kPa used by others over a 100 d operation period without cleaning.15,26 A rapid increase in the TMP in the AOFMBR was observed in phase 3, which usually would indicate sudden changes in the biofilm or cake layer structure.29 Sudden changes in TMP appear to occur more frequently in lab-scale membrane bioreactors than larger reactors.1,30 Even with this rapid TMP increase, the TMP of AOFMBR was still comparable to that of AFMBR. The initial TMP for AeMBR were higher than that of AFMBR and AOFMBR in both phase 1 and 4, when the membrane was replaced by fresh one. The reason for this difference was likely due to the absence of the GAC for membrane scouring in the AeMBR, compared to the other two reactors. The membrane flux of 11.6 L m−2-membrane-h in this study was close to that used in previous AFMBR studies (8–16 L m−2-membrane-h), and the TMP increase of the AFMBR in phases 1 and 2 was also consistent with previous reports.12–14,31 The membrane flux set here was within a range typical of AnMBRs (10–40 L m−2-membrane-h)6 and AeMBRs (4–36 L m−2-membrane-h).32
4.2. Microbial community analysis
Phylum-level microbial community analyses in the AFMBRs have not been previously reported, and therefore comparisons cannot be made to previous studies for this reactor or the new AOFMBR. However, comparisons are possible to communities in conventional treatment systems. At phylum level, the dominant four phyla, Proteobacteria, Bacteroidetes, Firmicutes, and Actinobacteria, in the suspended biomass in the AOFMBR and AeMBR were the same four phyla as those identified in a previous study of 15 activated sludge samples collected from 14 treatment plants.33 The phyla with relative abundance above 5% in the suspended biomass of the AFMBR, Proteobacteria, Bacteroidetes, Firmicutes, and Fusobacteria, were also found dominant in an anaerobic moving bed biofilm reactor treating municipal wastewater.34 This suggests that the microbial communities in suspension at the genera level, resembled those communities from other aerobic and aerobic/anaerobic (moving bed) systems.The GAC in the AFMBR and AOFMBR provides a more unique growth environment compared to the suspended cells. We observed a selective enrichment of Geobacter and certain methanogens (e.g. Methanothrix and Methanospirillum) in the GAC communities in the AFMBR. The abundance of these two groups on the GAC may be important, as Geobacter was shown to colonize GAC and conduct extracellular electron transfer to methanogens under anaerobic growth conditions.35,36 The transfer of extracellular electrons is particularly beneficial for acetoclastic methanogens as acetate has to be activated first at the expense of adenosine triphosphate (ATP) in order to generate methane and carbon dioxide.37 This benefit could help explain the higher relative abundance of Methanothrix, an acetoclastic methanogen, on the GAC in the AFMBR.
The biofilm on the GAC in the AOFMBR, had a greater abundance of Nitrospira and Nitrosomonas compared to the suspended microbial communities in either the AOFMBR or the AeMBR. This suggests that nitrifying bacterial populations may have benefited from growth conditions on the GAC. Unfortunately, the enriched abundance of these microorganisms was not determined until the completion of the study, and nitrogen balances were not conducted as a part of this study. The comparatively higher relative abundance of Nitrosomonas and Nitrospira on the GAC in the AOFMBR, as well as the low concentrations of COD in the reactor effluent, suggest that having GAC in this system might produce conditions favorable for nitrification.
The suspended microbial communities in the AOFMBR also had a higher relative abundance of methane-oxidizing bacteria (e.g. Methylomonas, Methylosarcina and Methylococcus) compared to those in the AFMBR. The presence of the methane-oxidizing bacteria in the AOFMBR could indicate that methanogenesis may have occurred in this system, or they could just reflect growth of cells on dissolved methane present in the influent wastewater. The possibility of methane oxidation in this system may be an interesting area for further study.
4.3. Energy consumption
Although methane gas production was avoided in the AOFMBR, energy consumption was 4 times greater than that of the AFMBR. However, the presence of the GAC in the AOFMBR resulted in better membrane fouling control and better COD removal than the AeMBR. The minimum aeration intensity needed to effectively fluidize the GAC (6 g, 92 g L−1) was 0.005 m3 m−2-cross section area-s, which is comparable to some AeMBRs.28,38,39 The energy consumed by the AOFMBR and AeMBR was therefore similar to that of an AeMBR (0.3–0.6 kW h m−3)6 and activated sludge process (0.3–0.6 kW h m−3).17 However, the energy needed for AFMBR operation estimated here (0.06 kW h m−3) and those reported by others (0.019 to 0.028 kW h m−3)12,13 was about one tenth of that used by other types of AnMBRs (0.25–1 kW h m−3).6 One reason for the lower energy requirements of an AFMBR is that water recirculation is less energy intensive that gas sparging. Additional headlosses, for example, due to an increase in viscosity with MLSS accumulation, was not included in the calculations for the AFMBR, and thus the actual energy for that system is underestimated relative to the gas sparging systems.Gas-phase methane produced in the AFMBR averaged 6.2 ± 1.2 L m−3-wastewater treated (0.02 m3-CH4 kg COD−1) in phases 3 and 4. Although methane production overall was low compared to a previous AFMBR study,14 it was 36 times as high as a previous study using MFC effluent with the same AFMBR reactor design. The increased gas production observed here was most likely due to the higher influent COD in phases 3 (299 ± 24 mg L−1) and 4 (329 ± 37 mg L−1) compared to the previous study13 of 107 ± 10 mg L−1. The methane production measured here, however, was 10 times lower than that typical of UASBs, CSTRs and AnMBRs treating various of wastewaters (0.2 to 0.4 m3-CH4 kg COD−1),40 which is a consequence of the different HRTs and influent CODs of AFMBR compared to these studies with other types of reactors.
It should be possible to reduce the energy used by the AOFMBR. Based on Stokes' law, using GAC with a smaller size could reduce the needed aeration intensity needed to fluidize the media, as the settling velocity is dependent on particle size squared. Also, particles other than GAC could be used, but these materials might not be good adsorbents. Larger media has been found to be more useful for reducing fouling than smaller media (particle sizes ranging from 0.2 to 2 mm).41 This suggests that there is an optimum particle size that can be chosen to balance energy demands with reduced membrane fouling. It might also be possible to use intermittent aeration in the AOFMBR, which has been shown to have better fouling control under some operational conditions in AeMBRs.1
5. Conclusions
An AOFMBR was developed by replacing recirculation in an AFMBR with aeration as the driving force for GAC fluidization, with a near-term goal of avoiding methane production and a long-term goal of reducing energy demands relative to AnMBRs and AeMBRs. Operation of the AOFMBR with two controls, the anaerobic AFMBR with GAC, and the AeMBR with aeration but no GAC, showed the following:1. The effluent COD in the AOFMBR was maintained at the lowest concentrations compared to the other two reactors, with average maximum removal efficiencies of 92% (COD) and 87% (SCOD) for the high influent CODs in the last two phases (averaging 299 ± 24 mg L−1 in phase 3, and 329 ± 37 mg L−1 in phase 4).
2. Effluent turbidity of the AOFMBR was steady and averaged 0.2 NTU, while the AFMBR had occasional spikes in the effluent turbidity.
3. Membrane fouling was better controlled in the AOFMBR under different influent CODs of 153 and 214 mg L−1, with a TMP increase rate of only 80% compared to the AeMBR, and 43% compared to the AFMBR. SEM images also supported less membrane fouling in the presence of the GAC particles.
4. Eliminating recirculation by using air bubble in the AOFMBR compared to recirculation in the AFMBR did not result in a lower energy consumption for the AOFMBR. The use of intermittent aeration or optimization of the size and density of the media that is fluidized in the reactor could contribute lowering energy costs for operation.
Acknowledgements
This work was supported by Strategic Environmental Research and Development Program (SERDP), and Award OSR-2015-SEED-2450-01 from the King Abdullah University of Science and Technology (KAUST). We also would like to thank John J. Cantolina, the technologist from Huck institute of the Life Sciences, the Pennsylvania State University for his help in SEM imaging.References
- F. Meng, S.-R. Chae, A. Drews, M. Kraume, H.-S. Shin and F. Yang, Water Res., 2009, 43, 1489–1512 CrossRef CAS PubMed.
- I. Chang, P. Le Clech, B. Jefferson and S. Judd, J. Environ. Eng., 2002, 128, 1018–1029 CrossRef CAS.
- K. Kimura, N. Yamato, H. Yamamura and Y. Watanabe, Environ. Sci. Technol., 2005, 39, 6293–6299 CrossRef CAS PubMed.
- B. Fan and X. Huang, Environ. Sci. Technol., 2002, 36, 5245–5251 CrossRef CAS PubMed.
- P. L. Mccarty, J. Bae and J. Kim, Environ. Sci. Technol., 2011, 45, 7100–7106 CrossRef CAS PubMed.
- B.-Q. Liao, J. T. Kraemer and D. M. Bagley, Crit. Rev. Environ. Sci. Technol., 2006, 36, 489–530 CrossRef CAS.
- G. Skouteris, D. Hermosilla, P. López, C. Negro and Á. Blanco, Chem. Eng. J., 2012, 198–199, 138–148 CrossRef CAS.
- K. Xie, H. J. Lin, B. Mahendran, D. M. Bagley, K. T. Leung, S. N. Liss and B. Q. Liao, Environ. Technol., 2010, 31, 511–521 CrossRef CAS PubMed.
- I. Vyrides and D. C. Stuckey, Water Res., 2009, 43, 933–942 CrossRef CAS PubMed.
- P. Sui, X. Wen and X. Huang, Desalination, 2008, 219, 203–213 CrossRef CAS.
- K.-H. Choo and C.-H. Lee, Water Res., 1998, 32, 3387–3397 CrossRef CAS.
- J. Kim, K. Kim, H. Ye, E. Lee, C. Shin, P. L. McCarty and J. Bae, Environ. Sci. Technol., 2011, 45, 576–581 CrossRef CAS PubMed.
- L. Ren, Y. Ahn and B. E. Logan, Environ. Sci. Technol., 2014, 48, 4199–4206 CrossRef CAS PubMed.
- R. Lee, P. L. Mccarty and J. Kim, J. Chem. Technol. Biotechnol., 2015, 90, 391–397 CrossRef CAS.
- J. Bae, C. Shin, E. Lee, J. Kim and P. L. Mccarty, Bioresour. Technol., 2014, 165, 75–80 CrossRef CAS PubMed.
- A. L. Smith, S. J. Skerlos and L. Raskin, Environ. Sci.: Water Res. Technol., 2015, 1, 56–64 CAS.
- M. D. Seib, K. J. Berg and D. H. Zitomer, Environ. Sci.: Water Res. Technol., 2016, 2, 290–297 CAS.
- K.-Y. Kim, W. Yang, Y. Ye, N. LaBarge and B. E. Logan, Bioresour. Technol., 2016, 208, 58–63 CrossRef CAS PubMed.
- H. Cheng, Y. Xie, L. F. Villalobos, L. Song, K.-V. Peinemann, S. Nunes and P.-Y. Hong, Sci. Rep., 2016, 6, 24289 CrossRef CAS PubMed.
- M. Harb, Y. Xiong, J. Guest, G. Amy and P.-Y. Hong, Environ. Sci.: Water Res. Technol., 2015, 1, 800–813 Search PubMed.
- A. L. Smith, L. B. Stadler, N. G. Love, S. J. Skerlos and L. Raskin, Bioresour. Technol., 2012, 122, 149–159 CrossRef CAS PubMed.
- J. Bae, R. Yoo, E. Lee and P. L. McCarty, Water Sci. Technol., 2013, 68, 394–399 CrossRef CAS PubMed.
- K. Brindle and T. Stephenson, Biotechnol. Bioeng., 1996, 49, 601–610 CrossRef CAS PubMed.
- Z. Ying and G. Ping, Sep. Purif. Technol., 2006, 52, 154–160 CrossRef CAS.
- K.-H. Choo and C.-H. Lee, Water Res., 1996, 30, 1771–1780 CrossRef CAS.
- C. Shin, P. L. Mccarty, J. Kim and J. Bae, Bioresour. Technol., 2014, 159, 95–103 CrossRef CAS PubMed.
- Y. Satyawali and M. Balakrishnan, J. Hazard. Mater., 2009, 170, 457–465 CrossRef CAS PubMed.
- M. A. H. Johir, R. Aryal, S. Vigneswaran, J. Kandasamy and A. Grasmick, J. Membr. Sci., 2011, 374, 121–128 CrossRef CAS.
- J. Zhang, H. C. Chua, J. Zhou and A. G. Fane, J. Membr. Sci., 2006, 284, 54–66 CrossRef CAS.
- L. Xu, G. Zhang, G. Yuan, H. Liu, J. Liu and F. Yang, RSC Adv., 2015, 5, 22533–22543 RSC.
- R. Yoo, J. Kim, P. L. McCarty and J. Bae, Bioresour. Technol., 2012, 120, 133–139 CrossRef CAS PubMed.
- M. Gander, B. Jefferson and S. Judd, Sep. Purif. Technol., 2000, 18, 119–130 CrossRef CAS.
- T. Zhang, M.-F. Shao and L. Ye, ISME J., 2012, 6, 1137–1147 CrossRef CAS PubMed.
- K. Biswas, M. W. Taylor and S. J. Turner, Appl. Microbiol. Biotechnol., 2014, 98, 1429–1440 CrossRef CAS PubMed.
- F. Liu, A.-E. Rotaru, P. M. Shrestha, N. S. Malvankar, K. P. Nevin and D. R. Lovley, Energy Environ. Sci., 2012, 5, 8982 CAS.
- A.-E. Rotaru, P. M. Shrestha, F. Liu, M. Shrestha, D. Shrestha, M. Embree, K. Zengler, C. Wardman, K. P. Nevin and D. R. Lovley, Energy Environ. Sci., 2014, 7, 408 CAS.
- M. S. M. Jetten, A. J. M. Stams and A. J. B. Zehnder, J. Bacteriol., 1989, 171, 5430–5435 CAS.
- R. Liu, X. Huang, Y. F. Sun and Y. Qian, Process Biochem., 2003, 39, 157–163 CrossRef CAS.
- T. Ueda, K. Hata, Y. Kikuoka and O. Seino, Water Res., 1997, 31, 489–494 CrossRef CAS.
- C. Visvanathan and A. Abeynayaka, Membrane Water Treatment, 2012, 3, 1–23 CrossRef.
- M. Aslam, P. L. McCarty, J. Bae and J. Kim, Sep. Purif. Technol., 2014, 132, 10–15 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ew00203j |
| This journal is © The Royal Society of Chemistry 2016 |