Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
cis-Conformation of indigo in the coordination complex (indigo-O,O)(Cp*CrIICl)†
Dmitri V.
Konarev
 *a,
Salavat S.
Khasanov
b,
Aleksey V.
Kuzmin
b,
Alexander F.
Shestakov
a,
Akihiro
Otsuka
cd,
Hideki
Yamochi
cd,
Gunzi
Saito
*ef and
Rimma N.
Lyubovskaya
a
*a,
Salavat S.
Khasanov
b,
Aleksey V.
Kuzmin
b,
Alexander F.
Shestakov
a,
Akihiro
Otsuka
cd,
Hideki
Yamochi
cd,
Gunzi
Saito
*ef and
Rimma N.
Lyubovskaya
a
aInstitute of Problems of Chemical Physics RAS, Chernogolovka, Moscow Region, 142432, Russia. E-mail: konarev3@yandex.ru
bInstitute of Solid State Physics RAS, Chernogolovka, Moscow Region, 142432, Russia
cDivision of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto 606-8502, Japan
dResearch Center for Low Temperature and Materials Sciences, Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan
eFaculty of Agriculture, Meijo University, 1-501 Shiogamaguchi, Tempaku-ku, Nagoya 468-8502, Japan. E-mail: gsaito@meijo-u.ac.jp
fToyota Physical and Chemical Research Institute, 41-1, Yokomichi, Nagakute, Aichi 480-1192, Japan
First published on 3rd October 2016
Abstract
The interaction of decamethylchromocene (Cp*2Cr) with indigo in the presence of a Cl− source yields the coordination complex (indigo-O,O)(Cp*CrIICl) (1) in which one Cp* ligand at chromium is substituted by indigo. Indigo adopts an unusual cis-conformation in 1, allowing the coordination of both indigo carbonyl groups to one CrII center. Complex 1 contains CrII with an S = 1 spin state and indigo0. At the same time, calculations show that an excited ionic state is positioned close to the neutral ground state, providing the appearance of intense low-energy NIR bands in the spectrum of 1 at 820 and 1002 nm attributed to metal-to-ligand charge transfer.
Indigo and its derivatives are well-known dyes now produced on a multi-ton scale.1 Recently, these dyes have found new applications as electronic materials2 and materials for rechargeable batteries.3 Indigo exists as a trans-isomer stabilized by intramolecular hydrogen C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H–N bonds.4 The existence of the cis-isomer of indigo was predicted a long time ago but until now this isomer of indigo has neither been isolated from natural sources nor been synthesized in a pure form most probably because of destabilization resulting from the repulsion of carbonyl dipoles.5 The cis-isomer is observed only for some indigo derivatives and leuco indigo under photoisomerization.6 The properties of indigo can be affected not only by chemical modification2d but also by the coordination of transition metals.6 Such complexes are generally obtained for deprotonated indigo in monoanionic (indigoH−) and dianionic states (indigo2−). In all these complexes, indigo also exhibits a trans-conformation.7 Only some “N-indigo” derivatives formed metal complexes in the cis-conformation8 but no information about the coordination complexes of unsubstituted cis-indigo has been found.
O⋯H–N bonds.4 The existence of the cis-isomer of indigo was predicted a long time ago but until now this isomer of indigo has neither been isolated from natural sources nor been synthesized in a pure form most probably because of destabilization resulting from the repulsion of carbonyl dipoles.5 The cis-isomer is observed only for some indigo derivatives and leuco indigo under photoisomerization.6 The properties of indigo can be affected not only by chemical modification2d but also by the coordination of transition metals.6 Such complexes are generally obtained for deprotonated indigo in monoanionic (indigoH−) and dianionic states (indigo2−). In all these complexes, indigo also exhibits a trans-conformation.7 Only some “N-indigo” derivatives formed metal complexes in the cis-conformation8 but no information about the coordination complexes of unsubstituted cis-indigo has been found.
This work involved the study of the interaction of indigo with decamethylchromocene (Cp*2Cr) and showed that indigo acts as a strong ligand able to substitute one of the pentamethylcyclopentadienyl ligands at chromium, forming the coordination complex (indigo-O,O)(Cp*CrCl) (1). Moreover, indigo adopts an unusual cis-conformation in this complex, thereby allowing the coordination of both carbonyl groups to the chromium center. Transformation of trans- to cis-indigo can be realized in the coordination sphere of a chromium atom.
Indigo shows weak acceptor properties and can be reduced to the radical anion at a redox potential of −0.75 V vs. Ag/AgCl (or −0.795 V vs. SCE).9 The reduction ability of Cp*2Cr, which has the first oxidation potential of −1.04 V vs. SCE,10 is enough to produce indigo˙−. Indeed, indigo completely dissolves in o-dichlorobenzene following the interaction with Cp*2Cr under strictly anaerobic conditions, and a red solution characteristic of indigo˙− (ref. 9) was formed. Slow mixing of the resultant solution with n-hexane did not yield any crystalline product. The addition of a stoichiometric amount of the chloro(1,5-cyclooctadiene)rhodium(I) dimer, {RhI(cod)Cl}2 (which was used to affect the crystallization), resulted in a color change of the solution from red to brown over several hours. Slow diffusion of n-hexane into this solution over the course of one month produced crystals as black parallelepipeds. The composition of these crystals determined from single crystal X-ray diffraction was (indigo-O,O)(Cp*CrCl) (1), i.e. (indigo-O,O)-chloro-(η5-pentamethylcyclopentadienyl)chromium(II).
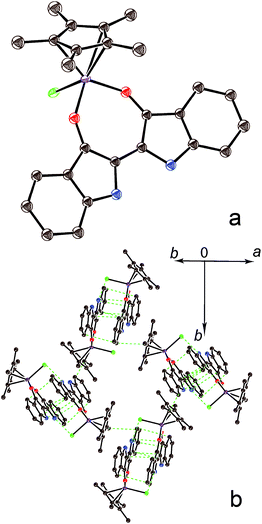
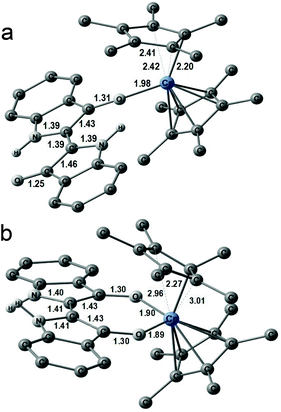
The crystal structure of 1 was studied at 150 K,‡ and the molecular structure of (indigo-O,O)(Cp*CrCl) is shown in Fig. 1a. The indigo molecule had a cis-conformation that allowed the coordination of both carbonyl groups to the chromium center. The length of the O(indigo)–Cr bonds was 1.911(2)–1.923(2) Å. The length of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds in 1 was 1.296(3) Å. These bonds are elongated in comparison with those in trans-indigo (1.239(2) Å).4a Previously, the elongation of the C
O bonds in 1 was 1.296(3) Å. These bonds are elongated in comparison with those in trans-indigo (1.239(2) Å).4a Previously, the elongation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds of indigo has been observed for the coordinated carbonyl in trans-indigo-Pd(Bu3P)Cl (1.276(3) Å), which is significantly longer than the C
O bonds of indigo has been observed for the coordinated carbonyl in trans-indigo-Pd(Bu3P)Cl (1.276(3) Å), which is significantly longer than the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond with a non-coordinated oxygen atom (1.225(3) Å).7a The central C
O bond with a non-coordinated oxygen atom (1.225(3) Å).7a The central C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond and the average C–N bond lengths in 1 were 1.400(4) and 1.392(3) Å, respectively, which were also elongated compared with those in trans-indigo (1.343(2) and 1.381(2) Å, respectively4a). These effects can be attributed to the coordination of indigo to chromium. The length of the Cr–Cl bond was 2.3948(7) Å and the average length of the Cr–C(Cp*) bonds was 2.247(2) Å. The chromium atom was positioned nearly in the plane of the indigo molecule (the displacement was only 0.020 Å). The cis conformation of the indigo molecule resulted in a non-parallel arrangement of the C
C bond and the average C–N bond lengths in 1 were 1.400(4) and 1.392(3) Å, respectively, which were also elongated compared with those in trans-indigo (1.343(2) and 1.381(2) Å, respectively4a). These effects can be attributed to the coordination of indigo to chromium. The length of the Cr–Cl bond was 2.3948(7) Å and the average length of the Cr–C(Cp*) bonds was 2.247(2) Å. The chromium atom was positioned nearly in the plane of the indigo molecule (the displacement was only 0.020 Å). The cis conformation of the indigo molecule resulted in a non-parallel arrangement of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds with a dihedral angle of 14.62(2)° between them. The cis-isomer has a slightly twisted shape with a dihedral C(O)–C–C–C(O) angle of 7.0° and the displacement of the oxygen atoms from the 18-atom indigo plane by 0.158 and 0.302 Å in opposite directions. The distance between the hydrogen atoms attached to nitrogen was only 2.146 Å in the cis-isomer, which is also rather short. These data indicate repulsion between the molecular permanent dipoles resulting from the orientation of the C
O bonds with a dihedral angle of 14.62(2)° between them. The cis-isomer has a slightly twisted shape with a dihedral C(O)–C–C–C(O) angle of 7.0° and the displacement of the oxygen atoms from the 18-atom indigo plane by 0.158 and 0.302 Å in opposite directions. The distance between the hydrogen atoms attached to nitrogen was only 2.146 Å in the cis-isomer, which is also rather short. These data indicate repulsion between the molecular permanent dipoles resulting from the orientation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups and intramolecular repulsion between the N–H hydrogen atoms in the cis-isomer.
O groups and intramolecular repulsion between the N–H hydrogen atoms in the cis-isomer.
The coordination units form {(indigo-O,O)(Cp*CrCl)}2 π-dimers in 1 (Fig. 1b) with a short interplanar distance of 3.44 Å and a parallel arrangement. As a result, multiple C,N⋯C,N van der Waals contacts are formed between indigo moieties (shown by green dashed lines in Fig. 1b). The Cp*CrCl groups are positioned on the opposite sides of the dimer and the shortest Cr⋯Cr distance was 8.01 Å. The π-dimers arranged in the layers were packed in such a way that the Cp* group formed weak π-stacks with the indigo molecule of the neighbouring dimer (Fig. 1b).
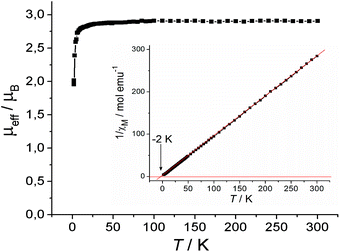
The magnetic properties of 1 were analysed using EPR spectroscopy and the SQUID technique. Charge transfer (CT) produced CrIII and indigo˙− species are generally well resolved in the EPR spectrum. The absence of any signal between room temperature and 4 K indicated the presence of an integer-spin system CrII that is EPR silent in X-band EPR.11 Indeed, the complex had an effective magnetic moment of 2.91 μB at 300 K (Fig. 2), close to the magnetic moment calculated for a system containing one non-interacting S = 1 spin from CrII (2.83 μB). The reciprocal molar magnetic susceptibility of 1 was linear in the 10–300 K range, showing the Weiss temperature of −2 K (Fig. 2, inset). This result indicates weak antiferromagnetic coupling of the spins which can also explain the decrease in the magnetic moment of 1 below 12 K (Fig. 2). The distance between the CrII atoms in the π-dimers is rather long and, accordingly, diamagnetic indigo molecules cannot effectively mediate intermolecular magnetic coupling.
The trans–cis-isomerization of neutral indigo in the individual state requires 59.5 kcal mol−1. The barrier for the isomerization can be reduced to 38.4 kcal mol−1 under electron transfer. According to the calculations (see ESI, Fig. S1 and S2†), the central C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of trans-indigo is elongated from 1.368 Å in the neutral state to 1.388 Å in the radical anion state to provide easier isomerization of indigo˙−. The isomerization of trans-indigo through the enol form has an even lower activation barrier of 25 kcal mol−1 (Fig. S3†).
C bond of trans-indigo is elongated from 1.368 Å in the neutral state to 1.388 Å in the radical anion state to provide easier isomerization of indigo˙−. The isomerization of trans-indigo through the enol form has an even lower activation barrier of 25 kcal mol−1 (Fig. S3†).
The possible way for the formation of 1 is shown in Fig. 3. According to the calculations, the haptotropic shift of one Cp* ring in the outer sphere (trans-indigo˙−)(Cp*2Cr+) complex allows the formation of an inner sphere complex of indigo with one coordinated carbonyl group (Fig. 4a). This complex is unstable since it has slightly higher energy by 6.1 kcal mol−1 than the energy of non-interacting trans-indigo and Cp*2Cr. The geometry of the indigo ligand in this complex corresponds well to the geometry of indigo˙−, and the spin density on the Cr atom is equal to 2.74. The trans–cis isomerization of the indigo ligand in the coordination sphere of the chromium atom leads to the formation of an additional Cr–O bond (Fig. 4b) under the η1-coordination of one Cp* ring and the 3.7 kcal mol−1 energy release. The calculated activation energy of such isomerization is equal to 25.1 kcal mol−1 (see the structure of the transition state in Fig. S4†). In contrast to the previous case, the (cis-indigo-O,O)(Cp*2Cr) complex is stable with respect to the non-interacting cis-indigo and Cp*2Cr, the energy gain being 14.2 kcal mol−1. We suppose that since only cis-indigo can form a stable complex with Cp*2Cr, namely this isomer is stabilized in the complex with chromium. A possible scenario of the formation of 1 is the substitution of the η1-Cp* ligand by chloride anions in the intermediate (cis-indigo-O,O)(Cp*2Cr) complex. The source of chloride anions is probably {RhI(cod)Cl}2 also used in the synthesis (Fig. 3). The η5-coordination Cp* ligand on the RhI center is energy favorable and may be the driving force of the substitution reaction. According to the calculations and magnetic data, 1 has a neutral indigo ligand. Thus, initially the ionic (trans-indigo˙−)(Cp*2Cr+) pair transforms to neutral complex 1 under the isomerization of trans-indigo and the exchange of the donor Cp*− ligand on the chloride anion.
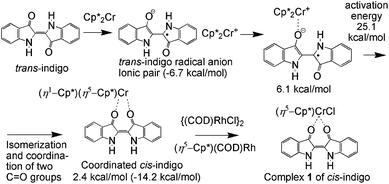 | ||
| Fig. 3 Possible route for the formation of 1, calculated relative energies are given with respect to the non-interacting Cp*2Cr and trans- or cis-indigo (in parenthesis). | ||
 | ||
| Fig. 4 (a) The calculated structure of [(trans-indigo-O)(Cp*2Cr)]; (a) and [(cis-indigo-O,O)(Cp*2Cr)]; (b) the interatomic distances are shown in Å. H atoms of C–H bonds are omitted for clarity. | ||
The IR spectrum of 1 is shown in Fig. S5† and the main peaks are listed in Table S1.† Coordination resulted in a decrease in the intensity of the bands attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibrations and their shift from 1613 and 1627 to 1607 and 1615 cm−1, respectively. The bands associated with the N–H vibrations in trans-indigo were observed at 3250 and 3270 cm−1 (Fig. S6a†). These bands were found in the spectrum of 1 at 3236 and 3295 cm−1 (Fig. S6b†), indicating the preservation of hydrogen at the nitrogen atoms. At the same time the essential shift of these bands can be attributed to the disappearance of the hydrogen C
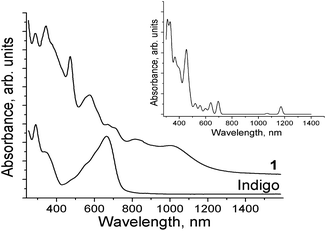
O vibrations and their shift from 1613 and 1627 to 1607 and 1615 cm−1, respectively. The bands associated with the N–H vibrations in trans-indigo were observed at 3250 and 3270 cm−1 (Fig. S6a†). These bands were found in the spectrum of 1 at 3236 and 3295 cm−1 (Fig. S6b†), indicating the preservation of hydrogen at the nitrogen atoms. At the same time the essential shift of these bands can be attributed to the disappearance of the hydrogen C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H–N bonds in the cis-conformation of indigo. The solid state spectra of indigo and 1 in the UV–visible–NIR range are shown in Fig. 5. Indigo exhibited bands at 334 and 291 nm and the lowest energy band is observed at 665 nm (the S0–S1 transition). The spectrum of 1 contained many bands with absorption up to 1250 nm. The bands of trans-indigo at 291 and 334 nm were reproduced in the spectrum of 1 at 285 and 342 nm, respectively, and a new band is manifested at 472 nm (Fig. 5). An intense band at 578 nm and weaker bands at 667 and 708 nm are also manifested in the spectrum of 1 (Fig. 5). The most interesting feature of the spectrum of 1 was the appearance of two relatively intense low-energy absorption bands with maxima at 820 and 1002 nm (Fig. 5). To understand the origin of these bands, the theoretical spectrum of 1 (Fig. 5, inset) was calculated using the TD-DFT approach based on the optimized structure (see the ESI†). The calculated spectrum showed a general resemblance to the experimental spectrum. According to the calculations, complex 1 with a triplet ground state (CrII, indigo0) has a low-lying excited ionic state located 1480 cm−1 above the ground state. The spin density on the Cr atom in this state is 2.75, corresponding to three unpaired electrons on chromium and an additional unpaired electron that was located on the indigo ligand, i.e. an excited quintet magnetic state is formed with the parallel orientation of the CrIII (S = 3/2) and indigo˙− (S = 1/2) spins. The corresponding triplet CT state with the antiparallel orientation of the spins should have a higher energy because of the effects of interactions with the ground triplet state. Thus, a spin-allowed triplet–triplet transition is expected to be observable in the NIR range of the spectrum of 1 as metal-to-ligand CT. The upper d-levels of the Cr complex have low splitting energies of 0.11–0.35 eV. This leads to the appearance of several CT transitions, namely NIR bands at 1066 and 1170 nm, located in the 800–1250 nm range of the experimental spectrum.
O⋯H–N bonds in the cis-conformation of indigo. The solid state spectra of indigo and 1 in the UV–visible–NIR range are shown in Fig. 5. Indigo exhibited bands at 334 and 291 nm and the lowest energy band is observed at 665 nm (the S0–S1 transition). The spectrum of 1 contained many bands with absorption up to 1250 nm. The bands of trans-indigo at 291 and 334 nm were reproduced in the spectrum of 1 at 285 and 342 nm, respectively, and a new band is manifested at 472 nm (Fig. 5). An intense band at 578 nm and weaker bands at 667 and 708 nm are also manifested in the spectrum of 1 (Fig. 5). The most interesting feature of the spectrum of 1 was the appearance of two relatively intense low-energy absorption bands with maxima at 820 and 1002 nm (Fig. 5). To understand the origin of these bands, the theoretical spectrum of 1 (Fig. 5, inset) was calculated using the TD-DFT approach based on the optimized structure (see the ESI†). The calculated spectrum showed a general resemblance to the experimental spectrum. According to the calculations, complex 1 with a triplet ground state (CrII, indigo0) has a low-lying excited ionic state located 1480 cm−1 above the ground state. The spin density on the Cr atom in this state is 2.75, corresponding to three unpaired electrons on chromium and an additional unpaired electron that was located on the indigo ligand, i.e. an excited quintet magnetic state is formed with the parallel orientation of the CrIII (S = 3/2) and indigo˙− (S = 1/2) spins. The corresponding triplet CT state with the antiparallel orientation of the spins should have a higher energy because of the effects of interactions with the ground triplet state. Thus, a spin-allowed triplet–triplet transition is expected to be observable in the NIR range of the spectrum of 1 as metal-to-ligand CT. The upper d-levels of the Cr complex have low splitting energies of 0.11–0.35 eV. This leads to the appearance of several CT transitions, namely NIR bands at 1066 and 1170 nm, located in the 800–1250 nm range of the experimental spectrum.
Most probably, the intense band at 578 nm corresponds to the S0–S1 transition in cis-indigo. It is noticeably blue shifted in comparison with the spectrum of trans-indigo (665 nm). The reasons for this can be trans–cis isomerization and/or deviation of the molecule from planarity in 1. It is known that the position of the lowest energy band in the indigo dyes is very sensitive to the conformation of the molecule and can be shifted to the blue side by 50 nm or more on trans–cis isomerization and/or deviation of the molecule from planarity.12 Indeed, the calculated S0–S1 transition in trans-indigo at 621 nm is shifted to 526 nm for cis-indigo or to 591 nm for the ‘frozen’ cis-indigo ligand of 1.
In conclusion, the preparation of coordination complex through the radical anions of indigo allows, for the first time, the observation of the cis-isomer of indigo, whose molecular structure is unknown yet. Up to now the cis-conformation has been observed only for some indigo derivatives.6cis-Indigo coordinates to chromium through two carbonyl groups in 1 and this coordination motif stabilizes the cis-isomer in spite of the fact that this isomer is less stable than trans-indigo. It should be noted that all previously studied transition metal complexes of indigo contain deprotonated trans-indigo anions.7 Some “N-indigo” derivatives can form metal complexes in both trans- and cis-conformations.8 Complex 1 is the first structurally characterized coordination complex of neutral cis-indigo. Though complex 1 has a neutral ground state, an excited charge transition state is positioned unusually close to provide the appearance of new intense NIR absorption bands in the spectrum of 1. The absorption of light in the NIR range can transfer this complex from the neutral to an excited ionic state which is characterized by a high-spin CrIII magnetic state. We believe that such complexes are promising materials for detectors and switchers operating in the NIR range. The range of transition metal complexes of cis-indigo can be extended by using indigo derivatives and by applying other metallocenes instead of Cp*2Cr. This work is now in progress.
This work was supported by JSPS KAKENHI grant numbers JP23225005 and JP26288035 and was partially supported by the JST(ACCEL)27(100150500010) project.
Notes and references
- (a) C. J. Cooksey, Biotech. Histochem., 2007, 82, 105 CrossRef CAS PubMed; (b) N. Stasiak, W. Kukuła-Koch and K. Głowniak, Acta Pol. Pharm., 2014, 71, 215 Search PubMed.
- (a) M. Irimia-Vlada, E. D. Głowacki, P. A. Troshin, G. Schwabegger, L. Leonat, D. K. Susarova, O. Krystal, M. Ullah, Y. Kanbur, M. A. Bodea, V. F. Razumov, H. Sitter, S. Bauer and N. S. Sariciftci, Adv. Mater., 2012, 24, 375 CrossRef PubMed; (b) E. D. Głowacki, G. Voss and N. S. Sariciftci, Adv. Mater., 2013, 25, 6783 CrossRef PubMed; (c) O. Pitayatanakul, T. Higashino, T. Kadoya, M. Tanaka, H. Kojima, M. Ashizawa, T. Kawamoto, H. Matsumoto, K. Ishikawa and T. Mori, J. Mater. Chem. C, 2014, 2, 9311 RSC; (d) V. Klimovich, L. I. Leshanskaya, S. I. Troyanov, D. V. Anokhin, D. V. Novikov, A. A. Piryazev, D. A. Ivanov, N. N. Dremova and P. A. Troshin, J. Mater. Chem. C, 2014, 2, 7621 RSC.
- (a) M. Yao, K. Kuratani, T. Kojima, N. Takeichi, H. Senoh and T. Kiyobayashi, Sci. Rep., 2014, 4, 3650 Search PubMed; (b) M. Yao, H. Sano, H. Ando and T. Kiyobayashi, Sci. Rep., 2015, 5, 10962 CrossRef CAS PubMed.
- (a) P. Süsse, M. Steins and V. Kupcik, Z. Kristallogr., 1988, 184, 269 CrossRef; (b) F. Kettner, L. J. Schäfer, K. Röder, U. Purgahn and H. Krautscheid, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2011, 67, o2867 CAS.
- B. T. Golding and C. Pierpoint, Educ. Chem., 1986, 23, 71 CAS.
- (a) E. C. Nicholls-Allison, G. Nawn, B. O. Patrick and R. G. Hicks, Chem. Commun., 2015, 51, 12482 RSC; (b) D. Farka, M. Scharber, E. D. Głowacki and N. S. Sariciftci, J. Phys. Chem. A, 2015, 119, 3563 CrossRef CAS PubMed; (c) R. Rondão, J. Seixas de Melo, M. J. Melo and A. J. Parola, J. Phys. Chem. A, 2012, 116, 2826 CrossRef PubMed.
- (a) W. Beck, C. Schmidt, R. Wienold, M. Steinmann and B. Wagner, Angew. Chem., Int. Ed. Engl., 1989, 28, 1529 CrossRef; (b) A. Lenz, C. Schmidt, A. Lehmann, B. Wagner and W. Beck, Z. Naturforsch., B: Chem. Sci., 1997, 52, 474 CAS; (c) J. Y. Wu, C. H. Chang, P. Thanasekaran, C. C. Tsai, T. W. Tseng, G. H. Lee, S. M. Peng and K. L. Lu, Dalton Trans., 2008, 6110 RSC; (d) P. Mondal, M. Chatterjee, A. Paretzki, K. Beyer, W. Kaim and G. K. Lahiri, Inorg. Chem., 2016, 55, 3105 CrossRef CAS PubMed.
- (a) G. Nawn, K. M. Waldie, S. R. Oakley, B. D. Peters, D. Mandel, B. O. Patrick, R. McDonald and R. G. Hicks, Inorg. Chem., 2011, 50, 9826 CrossRef CAS PubMed; (b) P. Mondal, S. Plebst, R. Ray, S. M. Mobin, W. Kaim and G. K. Lahiri, Inorg. Chem., 2014, 53, 9348 CrossRef CAS PubMed.
- A. Roessler, D. Crettenand, O. Dossenbach, W. Marte and P. Rys, Electrochim. Acta, 2002, 47, 1989 CrossRef CAS.
- J. L. Robbins, N. Edelstein, B. Spencer and J. C. Smart, J. Am. Chem. Soc., 1982, 104, 1882 CrossRef CAS.
- J. Telser, L. A. Pardi, J. Krzystek and L. C. Brunel, Inorg. Chem., 1998, 37, 5769 CrossRef CAS.
- W. Lüttke, H. Hermann and M. Klessinger, Angew. Chem., Int. Ed. Engl., 1966, 5, 598 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Materials, synthesis, details of theoretical calculations and X-ray diffraction experiment, IR spectra and data of magnetic measurements. CCDC 1493779. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6dt03545k |
‡ Crystal data for 1: C26H25ClCrN2O2, F.W. 484.93, black parallelepiped, 0.38 × 0.12 × 0.01 mm3, 150.0(2) K, monoclinic, space group P21/n, a = 11.7879(7), b = 15.2593(16), c = 12.5982(8) Å, β = 94.897(7)°, V = 2257.8(3) Å3, Z = 4, dcalcd = 1.427 g cm−3, μ = 0.651 mm−1, F(000) = 1008, 2θmax = 65.390°; 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 768 reflections collected, 7588 independent; R1 = 0.0548 for 4721 observed data [>2σ(F)] with 294 parameters; wR2 = 0.1442 (all data); final G.o.F. = 1.023. CCDC 1493779. 768 reflections collected, 7588 independent; R1 = 0.0548 for 4721 observed data [>2σ(F)] with 294 parameters; wR2 = 0.1442 (all data); final G.o.F. = 1.023. CCDC 1493779. |
| This journal is © The Royal Society of Chemistry 2016 |