Open Access Article
Open Access ArticleA drastic change in the superhydrophilic crystal porosities of metallosupramolecular structures via a slight change in pH†
Sireenart
Surinwong
,
Nobuto
Yoshinari
 ,
Tatsuhiro
Kojima
and
Takumi
Konno
*
,
Tatsuhiro
Kojima
and
Takumi
Konno
*
Department of Chemistry, Graduate School of Science, Osaka University, Toyonaka, Osaka 560-0043, Japan. E-mail: konno@chem.sci.osaka-u.ac.jp
First published on 14th October 2016
Abstract
A unique pH-controlled synthesis of two metallosupramolecular structures from CoIII2AuI3 complex anions and ZnII cations is reported. A dense coordination polymer (porosity ∼13%) was formed at a pH of 5.0, whereas a porous ionic framework (porosity ∼61%) that selectively adsorbs CO2 and H2O was created when the pH was adjusted to 5.5.
The preparation of porous crystalline materials such as metal–organic frameworks (MOFs), coordination polymers (CPs), and porous ionic frameworks has received continuous attention not only because of these materials’ fascinating structures but also because of their unique properties for gas storage and separation, chemical sensing, catalysis, etc.1–3 However, controlling the crystal porosities of this class of materials is frequently very difficult because of the effects of various synthesis parameters, including pH, solvent, and concentration, on the interactions between the metal ions and ligands.4 Therefore, the systematic synthesis of porous crystalline materials with the desired porosity by controlling these external factors remains an important research topic.
pH is one of the external stimuli that most strongly influence the construction of porous crystalline materials.5 This influence stems from the effect of changes in pH on the protonation level, which drastically changes the coordination modes and conformations of ligands, the geometry of metal centres, and the intermolecular hydrogen bonding interactions.5,6 Previous reports on the pH effect on the production of MOFs revealed that a higher pH tends to give frameworks with greater dimensionality compared to those formed at a lower pH.6 However, a systematic approach to controlling crystal porosities by alteration of pH has thus far been much less explored.
Herein, we report a unique metallosupramolecular system that exhibits a drastic increase in crystal porosity with an increase in the pH of reaction solutions. This system involves a dense coordination polymer, [Zn(H2O)4{Co2Au3(D-Hpen-N,S)(D-pen-N,S)5}] (1; porosity ∼13%, D-H2pen = D-penicillamine), and a highly porous ionic compound, Na9[Zn(OAc)2{Co2Au3(D-pen-N,S)6}2][Co2Au3(D-pen-N,S)6] (2; porosity ∼61%), both of which are independently produced from rod-shaped CoIII2AuI3 pentanuclear complex-anions [Co2Au3(D-pen-N,S)6]3−7 and Zn2+ cations at slightly different solution pH levels (5.0 vs. 5.5) (Scheme 1). To the best of our knowledge, such a drastic increase in crystal porosity from a dense coordination polymer to a porous ionic compound has not been previously reported.
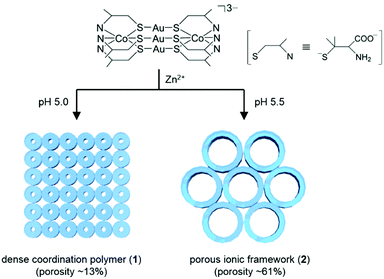 | ||
| Scheme 1 Synthetic routes and schematics of two different metallosupramolecular structures (1 and 2) constructed from [Co2Au3(D-pen-N,S)6]3− anions and Zn2+ cations at various pH values. | ||
Solutions of Na3[Co2Au3(D-pen-N,S)6]7 and Zn(OAc)2 in a sodium acetate buffer solution at pH 4.5 (HOAc/NaOAc = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were mixed together, yielding insoluble purple crystals with a square block shape (1).† The crystallization of 1 was complete within a day, with a yield of 58%. The diffuse reflection spectrum of 1 and the solid-state circular dichroism (CD) spectrum are similar to those of Na3[Co2Au3(D-pen-N,S)6], indicating that the S-bridged pentanuclear structure in [Co2Au3(D-pen-N,S)6]3− is retained in 1 (Fig. S1a and S2a).† The IR spectrum of 1 shows an intense C
1) were mixed together, yielding insoluble purple crystals with a square block shape (1).† The crystallization of 1 was complete within a day, with a yield of 58%. The diffuse reflection spectrum of 1 and the solid-state circular dichroism (CD) spectrum are similar to those of Na3[Co2Au3(D-pen-N,S)6], indicating that the S-bridged pentanuclear structure in [Co2Au3(D-pen-N,S)6]3− is retained in 1 (Fig. S1a and S2a).† The IR spectrum of 1 shows an intense C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching band at 1609 cm−1 with a shoulder at 1717 cm−1. The former and the latter correspond to deprotonated COO− and protonated COOH groups, indicative of the partial protonation of carboxylate groups in 1 (Fig. S3).†
O stretching band at 1609 cm−1 with a shoulder at 1717 cm−1. The former and the latter correspond to deprotonated COO− and protonated COOH groups, indicative of the partial protonation of carboxylate groups in 1 (Fig. S3).†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 X-ray fluorescence analysis confirmed the existence of Zn atoms, in addition to the Co and Au. Combining these results with the elemental analysis data, we predicted that 1 is a 1
8 X-ray fluorescence analysis confirmed the existence of Zn atoms, in addition to the Co and Au. Combining these results with the elemental analysis data, we predicted that 1 is a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adduct of Zn2+ and [Co2Au3(D-Hpen-N,S)(D-pen-N,S)5]2−, in which one of the six D-pen carboxyl groups is protonated.
1 adduct of Zn2+ and [Co2Au3(D-Hpen-N,S)(D-pen-N,S)5]2−, in which one of the six D-pen carboxyl groups is protonated.
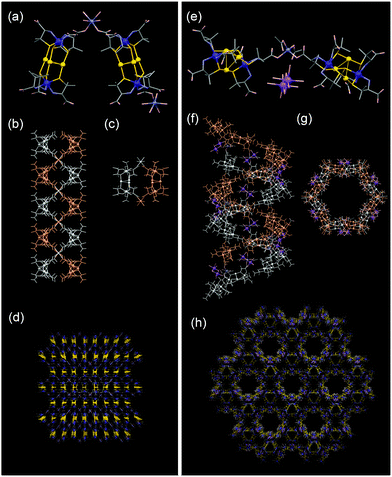
The structure of 1 was determined by single-crystal X-ray diffraction analysis. In addition to the water molecules of crystallization, 1 contains cis-configurational [Zn(H2O)4]2+ cations directly bound to [Co2Au3(D-Hpen-N,S)(D-pen-N,S)5]2− anions (Fig. 1a). In 1, [Co2Au3(D-Hpen-N,S)(D-pen-N,S)5]2− anions are alternately connected by the [Zn(H2O)4]2+ cations through coordination bonds (av. Zn–OOC = 2.02 Å), forming a 2-fold helix along the crystallographic a axis (Fig. 1b and c). Additionally, the two helices are intertwined and connected to each other through OH2⋯OOC hydrogen bonds (av. O⋯O = 2.72 Å), forming a tight right-handed double-helix structure (Fig. S4 and S5).† The double helices are connected to each other through COOH⋯OOC intermolecular hydrogen-bonding interactions (av. O⋯O = 2.84 Å) to form a 2D sheet-like structure (Fig. S6).† Finally, the 2D sheets are stacked through NH2⋯OOC hydrogen bonds (av. N⋯O = 2.98 Å), completing a 3D dense structure (Fig. 1d). The estimated solvent accessible volume of 1 was ∼13% on the basis of our calculations using the PLATON program.9
A similar treatment of Na3[Co2Au3(D-pen-N,S)6] and Zn(OAc)2 in a sodium acetate buffer solution at pH 5.0 (HOAc/NaOAc = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) also yielded only insoluble purple square block crystals of 1. New water-soluble purple hexagonal block crystals (2) were obtained when the pH of the buffer solution was increased to 5.5 (HOAc/NaOAc = 1
2) also yielded only insoluble purple square block crystals of 1. New water-soluble purple hexagonal block crystals (2) were obtained when the pH of the buffer solution was increased to 5.5 (HOAc/NaOAc = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6).†
6).†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ‡§ The diffuse reflection and solid-state CD spectra of 2 are essentially the same as those of 1 (Fig. S1b and S2b).† However, the IR spectrum displays a C
‡§ The diffuse reflection and solid-state CD spectra of 2 are essentially the same as those of 1 (Fig. S1b and S2b).† However, the IR spectrum displays a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching absorption only at 1611 cm−1 (Fig. S3), indicating that all carboxyl groups in 2 are deprotonated.†
O stretching absorption only at 1611 cm−1 (Fig. S3), indicating that all carboxyl groups in 2 are deprotonated.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 The X-ray fluorescence and elemental analysis results were in good agreement with a 1
8 The X-ray fluorescence and elemental analysis results were in good agreement with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 adduct of Zn2+ and [Co2Au3(D-pen-N,S)6]3−. In addition, 23Na and 1H NMR spectra revealed the presence of Na+ and OAc− ions, respectively (Fig. S7 and S8).† Single-crystal X-ray analysis revealed that 2 consists of tetrahedral {Zn(OAc)2} units, [Co2Au3(D-pen-N,S)6]3− anions, in addition to aqua Na+ cations and water molecules of crystallization (Fig. 1e). In 2, [Co2Au3(D-pen-N,S)6]3− anions are hydrogen bonded (av. N⋯O = 2.93 Å) to each other to construct a six-fold helix with right handedness along the c axis. The two helices are bridged by the {Zn(OAc)2} moieties (av. Zn–OOAc = 1.96 Å) through coordination bonds (av. Zn–Open = 1.97 Å), resulting in a tubular double helix structure with a large 1D pore with a diameter of ca. 20 Å (Fig. 1f and g). The double helices are further connected by other [Co2Au3(D-pen-N,S)6]3− anions via NH2⋯OOC hydrogen bonds (av. N⋯O = 2.91 Å), completing a 3D framework that possesses 1D pore channels (Fig. 1h and Fig. S9).† The channels accommodate water molecules and Na+ ions that are severely disordered. The estimated solvent accessible volume of 2 was ∼61%. This 1D channel structure is supported by aqua Na+ cations, each of which forms Na–OH2⋯OOC hydrogen bonds (av. O⋯O = 2.83 Å) between two [Co2Au3(D-pen-N,S)6]3− anions and one {Zn(OAc)2} unit in the double helix.
3 adduct of Zn2+ and [Co2Au3(D-pen-N,S)6]3−. In addition, 23Na and 1H NMR spectra revealed the presence of Na+ and OAc− ions, respectively (Fig. S7 and S8).† Single-crystal X-ray analysis revealed that 2 consists of tetrahedral {Zn(OAc)2} units, [Co2Au3(D-pen-N,S)6]3− anions, in addition to aqua Na+ cations and water molecules of crystallization (Fig. 1e). In 2, [Co2Au3(D-pen-N,S)6]3− anions are hydrogen bonded (av. N⋯O = 2.93 Å) to each other to construct a six-fold helix with right handedness along the c axis. The two helices are bridged by the {Zn(OAc)2} moieties (av. Zn–OOAc = 1.96 Å) through coordination bonds (av. Zn–Open = 1.97 Å), resulting in a tubular double helix structure with a large 1D pore with a diameter of ca. 20 Å (Fig. 1f and g). The double helices are further connected by other [Co2Au3(D-pen-N,S)6]3− anions via NH2⋯OOC hydrogen bonds (av. N⋯O = 2.91 Å), completing a 3D framework that possesses 1D pore channels (Fig. 1h and Fig. S9).† The channels accommodate water molecules and Na+ ions that are severely disordered. The estimated solvent accessible volume of 2 was ∼61%. This 1D channel structure is supported by aqua Na+ cations, each of which forms Na–OH2⋯OOC hydrogen bonds (av. O⋯O = 2.83 Å) between two [Co2Au3(D-pen-N,S)6]3− anions and one {Zn(OAc)2} unit in the double helix.
The spatial arrangement of [Co2Au3(D-pen-N,S)6]3− anions in 2 is reminiscent of that in the previously reported porous ionic crystal [Co(H2O)4][Co(H2O)6]2[Co2Au3(D-pen-N,S)6]2 (3).10 The substitution of linking trans-[Co(H2O)4]2+ units and free [Co(H2O)6]2+ ions in 3 by {Zn(OAc)2} units and [Na2(H2O)10]2+ ions in 2 is the only substantial structural difference between 2 and 3. However, the stabilities of 2 and 3 are quite different; a powder X-ray diffraction study demonstrated that 2 retains its crystallinity after being heated at 120 °C for 12 h, whereas 3 collapses immediately even at room temperature (Fig. S10 and S11).† As previously mentioned, multiple hydrogen bonds exist between acetate groups from {Zn(OAc)2} and aqua ligands in [Na2(H2O)10]2+ units in 2 (Fig. S12).† By contrast, no direct hydrogen-bonding interactions were observed between trans-[Co(H2O)4]2+ units and free [Co(H2O)6]2+ ions in 3, which is most likely the reason for the substantial difference in stability between 2 and 3.
The protonation and deprotonation of the carboxylate group at different pH values and in the presence/absence of an excess of Na+ and OAc− ions in reaction solutions are potential causes of the drastic change in crystal porosity between 1 and 2. In 1, the protonated D-pen carboxyl groups form strong intermolecular COOH⋯OOC hydrogen bonds, which bring neighbouring helices much closer. Consequently, a dense structure with low porosity (∼13%) was reasonably constructed. In this case, the presence of both the protonated and the deprotonated carboxylate groups in each complex anion in the pH range from 4.0 to 5.0 is reasonable.11 By contrast, a slight but higher pH value of 5.5 leads to a fully deprotonated form of D-pen in 2. Unlike in 1, Na+ and OAc− ions from the employed NaOAc buffer solution were incorporated into 2. Both Na+ and OAc− ions appear to be associated with the template-directed synthesis of 2, i.e. these ions form strong intermolecular hydrogen bonds to stabilize the 1D channel structure with substantially increased porosity. Presumably, the formation of 2 was accomplished with the aid of the stabilizing effect due to a large amount of Na+ and OAc− ions in a basic acetate buffer solution. However, we observed that 1 was also selectively produced using controlled experiments in the pH range from 4.0 to 5.0 when the reaction solutions contained the same amounts of Na+ and OAc− ions as those of 2 (Fig. S13).† This is because a large part of OAc− ions exist in a protonated form in this pH range to prevent the coordination to a Zn2+ centre. Thus, we concluded that pH is the dominant factor controlling the crystal porosities in the present metallosupramolecular system, changing the protonation/deprotonation states of the carboxylate groups.
The gas adsorption properties of 1 and 2 were investigated. As shown in Fig. S14, the CO2 adsorption isotherm for 1 at 195 K displayed a type-I physical sorption isotherm,12 showing a gradual increase to 8.7 cm3 g−1 at P/P0 = 0.99, with a low calculated BET surface area of 16 m2 g−1.† A similar CO2 adsorption isotherm was observed for 2; however, the adsorption amounts increased to a saturation value of 29.3 cm3 g−1 at P/P0 = 0.99 because of its higher porosity. The BET surface area calculated from the CO2 adsorption isotherm was also substantially greater: 66 m2 g−1. By contrast, the adsorption capacities of N2 gas for both compounds (Fig. S15 and S16) were very poor at 77 K (<5.0 cm3 g−1).† The adsorption properties toward small vapour molecules were also investigated. Although the degree of water molecule adsorption for 1 was quite small (5 mol mol−1 at P/P0 = 0.90), 2 exhibited an impressively high adsorption of 134 mol mol−1 (584 cm3 g−1) at P/P0 = 0.90 (Fig. 2). This adsorption value is comparable to those for MOFs with excellent water adsorption ability.¶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13,14 Notably, the ionic compound 2 possesses a substantial advantage over the MOFs that are commonly insoluble in solution because it can be regenerated via a dissolution–crystallization process. In an adsorption–desorption cycle, a large hysteresis loop was observed for 2, which is typical for nanoporous materials with large pores.14 Remarkably, not only 1 but also 2 exhibited no adsorption ability toward EtOH and acetone molecules (Fig. S17 and S18), reflecting the superhydrophilic character of their porous structures surrounded by numerous hydrophilic groups.†
13,14 Notably, the ionic compound 2 possesses a substantial advantage over the MOFs that are commonly insoluble in solution because it can be regenerated via a dissolution–crystallization process. In an adsorption–desorption cycle, a large hysteresis loop was observed for 2, which is typical for nanoporous materials with large pores.14 Remarkably, not only 1 but also 2 exhibited no adsorption ability toward EtOH and acetone molecules (Fig. S17 and S18), reflecting the superhydrophilic character of their porous structures surrounded by numerous hydrophilic groups.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 Previously, similar superhydrophilic behaviour was observed for 3. However, the adsorption capacity of 2 is much greater than that of 3 (33 mol mol−1 at P/P0 = 0.90), which is ascribed to the very rigid porous framework in 2.
15 Previously, similar superhydrophilic behaviour was observed for 3. However, the adsorption capacity of 2 is much greater than that of 3 (33 mol mol−1 at P/P0 = 0.90), which is ascribed to the very rigid porous framework in 2.
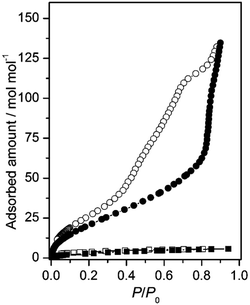 | ||
| Fig. 2 Comparison of H2O adsorption (solid symbols) and desorption (open symbols) isotherms at 298 K for 1 (–■–) and 2 (–●–). | ||
In summary, we demonstrated that two metallosupramolecular compounds (1 and 2) of remarkably different porosities (∼13% and ∼61%, respectively) can be independently created from [Co2Au3(D-pen-N,S)6]3− anions and Zn2+ cations with only a slight change in solution pH (5.0 vs. 5.5). Such a drastic change in crystal porosities by a slight change in pH has not been previously reported for crystalline coordination compounds. The formation of stable, superhydrophilic opening channels in 2, which are applicable for the selective inclusion of hydrophilic small molecules, is noteworthy. This study should serve as a guide for further development of the synthesis of reproducible, functional porous materials consisting of cationic and anionic species.
This work was supported by CREST, JST and JSPS KAKENHI Grant Number 15K21127 and 16K13609. The synchrotron radiation experiments were performed at the BL02B1 and BL02B2 beamlines of SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal No. 2015B1001, 2015B1237, 2016A1073) and at the 2D beamline in the Pohang Accelerator Laboratory supported by POSTECH.
Notes and references
- (a) S. Kitagawa, R. Kitaura and S. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334 CrossRef CAS PubMed; (b) J. R. Li, R. J. Kuppler and H. C. Zhou, Chem. Soc. Rev., 2009, 38, 1477 RSC; (c) R. Eguchi, S. Uchida and N. Mizuno, Angew. Chem., Int. Ed., 2012, 51, 1635 CrossRef CAS PubMed; (d) R. Eguchi, S. Uchida and N. Mizuno, J. Phys. Chem. C, 2012, 116, 16105 CrossRef CAS; (e) S. Uchida, R. Kawahara, Y. Ogasawara and N. Mizuno, Dalton Trans., 2013, 42, 16209 RSC; (f) H. Furukawa, K. E. Cordova, M. O’Keeffe and O. M. Yaghi, Science, 2013, 341, 974 CrossRef CAS PubMed; (g) Y. Mito-oka, Y. Sawada, T. Masumori, S. Horike, H. Kitagawa and S. Kitagawa, Chem. Lett., 2015, 44, 1694 CrossRef CAS.
- (a) L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105 CrossRef CAS PubMed; (b) Y.-W. Li, J.-R. Li, L.-F. Wang, B.-Y. Zhou, Q. Chen and X.-H. Bu, J. Mater. Chem. A, 2013, 1, 495 RSC; (c) Z. Dou, J. Yu, Y. Cui, Y. Yang, Z. Wang, D. Yang and G. Qian, J. Am. Chem. Soc., 2014, 136, 5527 CrossRef CAS PubMed.
- (a) N. Guillou, Q. Gao, P. M. Forster, J. S. Chang, M. Noguès, S. E. Park, G. Férey and A. K. Cheetham, Angew. Chem., Int. Ed., 2001, 40, 2831 CrossRef CAS; (b) A. Proust, R. Thouvenot and P. Gouzerh, Chem. Commun., 2008, 1837 RSC; (c) J. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450 RSC; (d) Z.-J. Liu, S. Yao, Z.-M. Zhang and E.-B. Wang, RSC Adv., 2013, 3, 20829 RSC; (e) R. Kawahara, K. Niinomi, J. N. Kondo, M. Hibino, N. Mizuno and S. Uchida, Dalton Trans., 2016, 45, 2805 RSC.
- (a) N. Stock and S. Biswas, Chem. Rev., 2012, 112, 933 CrossRef CAS PubMed; (b) D. Kim, X. Song, J. H. Yoon and M. S. Lah, Cryst. Growth Des., 2012, 12, 4186 CrossRef CAS; (c) T. Liu, D. Luo, D. Xu, H. Zeng and Z. Lin, Inorg. Chem. Commun., 2013, 29, 110 CrossRef CAS; (d) P.-Z. Li, X.-J. Wang, Y. Li, Q. Zhang, R. H. D. Tan, W. Q. Lim, R. Ganguly and Y. Zhao, Microporous Mesoporous Mater., 2013, 176, 194 CrossRef CAS; (e) Y.-X. Sun and W.-Y. Sun, Chin. Chem. Lett., 2014, 25, 823 CrossRef CAS.
- (a) R.-Q. Zhong, R.-Q. Zou, M. Du, T. Yamada, G. Maruta, S. Takeda and Q. Xu, Dalton Trans., 2008, 2346 RSC; (b) H. Wang, Y.-Y. Wang, G.-P. Yang, C.-J. Wang, G.-L. Wen, Q.-Z. Shi and S. R. Batten, CrystEngComm, 2008, 10, 1583 RSC; (c) B. Zheng, J. Bai and Z. Zhang, CrystEngComm, 2010, 12, 49 RSC; (d) J.-X. Yang, X. Zhang, J.-K. Cheng, J. Zhang and Y.-G. Yao, Cryst. Growth Des., 2012, 12, 333 CrossRef CAS; (e) H.-N. Wang, G.-S. Yang, X.-L. Wang and Z.-M. Su, Dalton Trans., 2013, 42, 6294 RSC; (f) K. P. Rao, M. Higuchi, J. Duan and S. Kitagawa, Cryst. Growth Des., 2013, 13, 981 CrossRef CAS; (g) C. Patzschke, C. M. Forsyth, S. R. Batten and A. L. Chaffee, CrystEngComm, 2014, 16, 6296 RSC.
- (a) S.-T. Wu, L.-S. Long, R.-B. Huang and L.-S. Zheng, Cryst. Growth Des., 2007, 7, 1746 CrossRef CAS; (b) R.-G. Lin, L.-S. Long, R.-B. Huang and L.-S. Zheng, Inorg. Chem. Commun., 2007, 10, 1257 CrossRef CAS; (c) Q. Chu, G.-X. Liu, T. Okamura, Y.-Q. Huang, W.-Y. Sun and N. Ueyama, Polyhedron, 2008, 27, 812 CrossRef CAS; (d) M. Kouno, Y. Miyashita, N. Yoshinari and T. Konno, Chem. Lett., 2015, 44, 1512 CrossRef CAS.
- T. Konno, A. Toyota and A. Igashira-Kamiyama, J. Chin. Chem. Soc., 2009, 56, 26 CrossRef CAS.
- K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, Wiley, Chichester, 5th edn, 1997 Search PubMed.
- A. L. Spek, J. Appl. Crystallogr., 2003, 36, 7 CrossRef CAS.
- S. Surinwong, N. Yoshinari, B. Yotnoi and T. Konno, Chem. – Asian J., 2016, 11, 486 CrossRef CAS PubMed.
- N. Yoshinari, K. Tatsumi, A. Igashira-Kamiyama and T. Konno, Chem. – Eur. J., 2010, 16, 14252 CrossRef CAS PubMed.
- K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquerol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603 CrossRef CAS.
- H. Furukawa, F. Gándara, Y.-B. Zhang, J. Jiang, W. L. Queen, M. R. Hudson and O. M. Yaghi, J. Am. Chem. Soc., 2014, 136, 4369 CrossRef CAS PubMed.
- (a) J. Canivet, A. Fateeva, Y. Guo, B. Coasne and D. Farrusseng, Chem. Soc. Rev., 2014, 43, 5594 RSC; (b) N. C. Burtch, H. Jasuja and K. S. Walton, Chem. Rev., 2014, 114, 10575 CrossRef CAS PubMed.
- (a) A. Nalaparaju, X. S. Zhao and J. W. Jiang, J. Phys. Chem. C, 2010, 114, 11542 CrossRef CAS; (b) A. Kobayashi, A. Sugiyama, T. Ohba, Y. Suzuki, H.-C. Chang and M. Kato, Chem. Lett., 2014, 43, 1070 CrossRef CAS; (c) J. J. Gutiérrez-Sevillano, S. Calero and R. Krishna, J. Phys. Chem. C, 2015, 119, 3658 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Details of syntheses together with spectroscopic data, adsorption data, PXRD results, and single-crystal X-ray diffraction data. CCDC 1500233 and 1500234. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6cc06943f |
| ‡ Similar reactions in a sodium acetate buffer solution at pH 6.0 and 6.5 also gave 2. |
| § When 1 was dissolved in a sodium acetate buffer solution of pH 5.5, 2 was selectively crystallized after several days. The reverse conversion from 2 into 1 also occurred when a sodium acetate buffer solution of pH 5.0 was employed.† |
| ¶ Although the water adsorption amount of 2 is lower than the reported record for MOFs (PIZOF-2; 850 cm3 g−1),13 this is the highest value for a porous ionic framework. |
| This journal is © The Royal Society of Chemistry 2016 |