Controlled synthesis of multi-armed P3HT star polymers with gold nanoparticle core†
Hyun-Ji Kimab,
Kie Yong Choa,
Seung Sang Hwangaf,
Dong Hoon Choib,
Min Jae Kocef and
Kyung-Youl Baek*adf
aMaterials Architecturing Research Center, Korea Institute of Science and Technology, Seoul 02792, Korea. E-mail: baek@kist.re.kr
bDepartment of Chemistry, Korea University, Seoul 02841, Korea
cPhoto-electronic Hybrids Research Center, Korea Institute of Science and Technology, Seoul 02792, Korea
dKIST-UNIST-Ulsan Center for Convergence Materials, Ulsan 689-798, Korea
eKU-KIST Graduate School of Converging Science and Technology, Korea University, Seoul, 02841, Republic of Korea
fNanomaterials Science and Engineering, University of Science and Technology, Daejeon 34113, Korea
First published on 4th May 2016
Abstract
Well-defined multi-armed P3HT star polymers with a gold nanoparticle (NP) core were synthesized by an arm-first method based on a ligand exchange reaction between linear end-functionalized P3HT (P3HT-SH) and gold NPs. A high loading amount of gold NPs to P3HT-SH with a relatively lower molecular weight gave a higher yield of star polymers (∼70%) with a high molecular weight (Mw = 2867k, PDI = 2.1), and the number of P3HT arm chains on one gold NP was 119. The P3HT star polymer with a gold NP core was well-dispersed both in solution and in solid, which was interestingly not crystalline because of the unique 3-dimenstional structure. In addition, surface plasmon resonance (SPR) absorption from the gold NP, as the core of the star polymer, was more enhanced both in solution and in solid, in comparison to those with non end-functionalized P3HT arm chains (P3HT-allyl); however, PL emission was more diminished because of the molecularly contacted P3HT arm chain and gold NP core. This was then introduced in an active layer consisting of P3HT:PCBM in an organic solar cell to increase optical absorption by the SPR effect from the gold NP, however, the device efficiency was rather decreased compared to that of the reference device without gold NPs, which was probably due to direct electron transfer between the gold NP and P3HT.
1. Introduction
Poly(3-hexyl thiophene) (P3HT) is one of the well-known conducting polymers, which has been applied in many organic electronic applications, such as organic photovoltaic cells (OPV),1 organic thin film transistors (OTFT),2 organic light emission diodes (OLED)3 and e-skin,4 because of its relatively high charge mobility, due to well-arrayed molecular complexes through pi–pi stacking.1–5 Recently developed as a controlled polymerization method, the Grignard metathesis method (GRIM) is especially interesting to give well-defined P3HT with controlled molecular weights and narrow polydispersity.6,7 However, synthesis with high molecular weight is still difficult because of the problem of solubility of the obtained P3HT in the reaction solvent, such as THF.8 Star polymers with high molecular weights often show better solubility than corresponding linear polymers, because their compact 3-dimensional structures give a lower radius of gyration in the polymer chains.9 The synthesis of star polymers is generally achieved by core and arm-first methods. The core-first method generally gives a well-defined number of arm chains of the star polymer because the polymer arm chains are grown from pre-determined initiating species, such as a multi-functionalized initiator. However, the number of arm chains is limited.10–12 Star polymers prepared by the arm-first method, which can be obtained by a crosslinking reaction of pre-synthesized linear polymers with a crosslinker (such as a divinyl compound, etc.), give large numbers of arm chains with a microgel core.9 Although the design of a multi-functional initiator in GRIM was difficult, a well-defined P3HT star polymer using the core-first method was recently developed,11 however, the obtained molecular weight was still low (Mw < 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). Recently, we reported multi-armed star polymers synthesized by the arm-first method by a combination of GRIM and atom transfer radical polymerization (ATRP).13 In detail, the linear P3HTs were first prepared by GRIM, followed by the introduction of an alkyl halide initiator into the end group of P3HT for ATRP, which was then reacted with an ethylene glycol dimethacrylate (EGDMA) crosslinker to give a P3HT star polymer with a microgel core. The obtained P3HT star polymer showed a high molecular weight (Mw > 1
000). Recently, we reported multi-armed star polymers synthesized by the arm-first method by a combination of GRIM and atom transfer radical polymerization (ATRP).13 In detail, the linear P3HTs were first prepared by GRIM, followed by the introduction of an alkyl halide initiator into the end group of P3HT for ATRP, which was then reacted with an ethylene glycol dimethacrylate (EGDMA) crosslinker to give a P3HT star polymer with a microgel core. The obtained P3HT star polymer showed a high molecular weight (Mw > 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). However, a relatively large amount of unreacted linear P3HT remained after the crosslinking reaction. As other approaches for the development of a P3HT star polymer, combinations of the core-first and the arm-first methods were also reported, with clickable cores and linear arm polymers being synthesized separately, followed by coupling through a click reaction to give a star polymer. However, such coupling reactions generally remained with the linear polymers being unreacted, because of difficulties with the stoichiometry.14,15
000). However, a relatively large amount of unreacted linear P3HT remained after the crosslinking reaction. As other approaches for the development of a P3HT star polymer, combinations of the core-first and the arm-first methods were also reported, with clickable cores and linear arm polymers being synthesized separately, followed by coupling through a click reaction to give a star polymer. However, such coupling reactions generally remained with the linear polymers being unreacted, because of difficulties with the stoichiometry.14,15
Gold nanoparticles (NPs) possess unique optical electronic properties such as surface plasmonic effects, and are now employed in many applications, such as in organic photovoltaics,16 sensory probes,17 therapeutic agents,18 drug delivery,19 electronic conductors,20 and catalysis.20,21 A gold NP is generally stabilized by small or oligomeric ligands including oxygen,22 amine,23 phosphine,24 and sulfur groups.25 Polymeric ligands are rarely reported because of the difficult controllability of the shape and size of gold NPs.26,27 Thus, the synthesis of gold NPs with a polymeric ligand is instead carried out by a post reaction, such as a ligand exchange reaction using a thiol-terminated polymer, which replaces the small or oligomeric ligands (such as oleylamine and ascorbate etc.) with thiol-terminated polymers, leading to better stability of the original shape and size of the gold NPs. The obtained product forms 3-dimensional structures called star polymers, with polymer arm chains radiating from a gold NP core, which will increase the dispersion stability in solution to result in better dispersion in a solid (matrix) as well.
Conducting polymers (CPs) with gold NPs have also been extensively studied, because the surface plasmonic effect from the gold NP significantly affects the electrical and optical properties of CPs,28,29 which are thus applied in many organic electronics such as sensors,30 solar cells,31 emitting diodes etc.32 to increase their efficiencies. The incorporation of gold NPs into CPs has been generally prepared by the in situ growth of gold NPs from a HAuCl4 precursor in the presence of CPs, which often include functional groups interacting with the precursor.33–37
However, the obtained gold NPs in CPs gave uncontrolled size and poor dispersion, because the employed CPs did not play the role of an efficient surfactant well. This can be expected due to the uncontrolled molecular weights and functional groups in the polymer chains.38,39 The ligand exchange method with thiol end-functionalized CPs is well-known and suitable to overcome such problems. However, this approach is still not easy because of the complicated synthetic methods required to introduce thiol groups into the selective position of CPs with controlled molecular weights. In a recent report, Scherman et al. demonstrated a ligand exchange method using a thiol terminated conducting polymer and various nanoparticles such as gold, silver, and quantum dots (QD).38 However, the employed conducting polymer was not a conjugated backbone polymer, but a random coil polymer with a small conjugated pendant group. In this study, GRIM was introduced as a controlled polymerization method to prepare a well-defined conjugated polymer such as P3HT, followed by end group-functionalization to give thiol-terminated P3HT,40 and then a ligand exchange reaction was carried out for the substitution of the oleylamine ligand on the surface of the gold NP with thiol-terminated P3HT. This arm-first method gave multi-armed P3HT star polymers with a gold NP core, where the crosslinker was changed from the conventional divinyl compound to the gold NP (Scheme 1). The conversions from the linear to the star polymer (yield) were systematically examined and the number of the arm chains per gold NP core upon varying the mole ratio of P3HT-SH to gold NP and the molecular weight of P3HT-SH were characterized. These ligand exchange reactions with gold NPs were also compared using non-functionalized P3HT. The obtained P3HT star polymers with gold NPs were then characterized to determine their morphological, thermal, electrical and optical properties. Notably, these were preliminarily applied for organic solar cells based on P3HT/PCBM as an active layer to examine the surface plasmonic effects of the gold NPs, because the good compatibility of the P3HT-coated gold NPs with the P3HT/PCBM active layer can give better dispersion without aggregation of gold NPs in the active layer, which might increase the device efficiency further.41
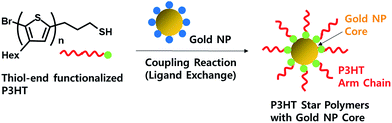 | ||
| Scheme 1 Synthesis of multi-armed P3HT star polymers with a gold NP core by the arm-first method, based on a ligand exchange reaction between linear P3HT-SH and gold NPs. | ||
2. Experimental
Materials
2,5-Dibromo-3-hexylthiophene was synthesized according to the published literature. The following chemicals were purchased from Sigma-Aldrich, and used without further purification: [1,3-bis(diphenylphosphino)propane]dichloronickel(II) (Ni(dppp)Cl2, 98%), 2,5-dibromo-3-hexylthiophene, tert-butyl magnesium chloride (2.0 M in diethyl ether), allylmagnesium bromide (1.0 M in diethyl ether), 9-borabicyclo[3.3.1]nonane (9-BBN, 98%), tributylamine (TBA, >98.5%), triethylamine (TEA, 99.5%), triphenylphosphine (≥95.0%), diisopropyl azodicarboxylate (DIAD, >98%), thioacetic acid (>96%), lithium aluminium hydride (1.0 M in THF solution), gold(III) chloride trihydrate (HAuCl4·3H2O, >99.9%), and oleylamine (>98%). Sodium hydroxide (NaOH) and hydrogen peroxide (H2O2, >30%) were purchased from Dae-Jung chemical and used as received. The following solvents were purchased from J. T. Baker (HPLC grade) and used as received: monochlorobenzene (MCB), chloroform, methanol, and n-hexane. THF was purchased from J. T. Baker (HPLC grade) and was dried overnight over CaH2, followed by distillation with Na-benzophenone. All reactions were performed inside a glove box (H2O < 0.1 ppm and O2 < 0.1 ppm). A series of end-functionalized P3HTs (P3HT-SH and P3HT-allyl) and gold NPs were synthesized according to the published literatures (see ESI†).Synthesis of P3HT star polymers with gold NP core
A typical synthesis with 25/75 wt% of P3HT-SH (Mw(MALDI) = 4.1k) and gold NPs (density = 19.3 g cm−3) is as follows: 1 mL of P3HT-SH solution (1.21 μM in THF) and 3 mL of gold NP solution (0.478 μM) were sequentially added to a 25 mL round bottom flask under Ar and stirred overnight at 25 °C. The obtained solution was directly used for SEC analysis (see Table 1 for final concentrations). In addition, the solutions were quantitatively diluted for optical analysis.| Run | P3HT (μM) | Gold NP (μM) | P3HT/gold NP (wt%) | Mw,SEC (×103) | PDI | Yielda (%) | Arm numberb |
|---|---|---|---|---|---|---|---|
| a Yield of star polymer = area (SEC) of unreacted P3HT-SH in the presence of gold NP/area (SEC) of P3HT-SH in the absence of gold NPs.b Number of P3HT arm chains = (molarity of P3HT × yield)/molarity of gold NP. | |||||||
| 1 | 60.98 | 0.03 | 80/20 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 981 981 |
1.6 | 43 | 887 |
| 2 | 60.98 | 0.06 | 67/33 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 251 251 |
1.7 | 46 | 469 |
| 3 | 60.98 | 0.12 | 50/50 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 422 422 |
1.8 | 59 | 301 |
| 4 | 60.98 | 0.24 | 34/66 | 3124 | 2.1 | 70 | 124 |
| 5 | 60.98 | 0.36 | 25/75 | 2867 | 2.1 | 70 | 119 |
Polymer characterization
The number average molecular weight (Mn) and molecular weight distributions (Mw/Mn) of the polymers were measured by a JASCO PU-2080 plus SEC system equipped with a differential RI-2031 detector and a UV-2057 detector (250 nm detection wavelength) using THF as the mobile phase at 40 °C and a flow rate of 1 mL min−1. The samples were separated through four columns (Shodex; KF-802, KF-803, KF-804, and KF-805). Calibration based on polystyrene standards was applied for determination of the molecular weights and polydispersity of the polymers. MALDI-TOF-MASS was performed using a Voyager-DE STR workstation by Applied Biosystems. All spectra were recorded using linear ion mode, in which samples were irradiated under high vacuum using a nitrogen laser (wavelength 337 nm, 2 ns pulse). 1H NMR spectra were recorded in CDCl3 at 25 °C on a Varian Gemini NMR (400 MHz) spectrometer. DSC was measured with a TA instrument Q-20 under nitrogen. TGA was measured with a TA instrument TGA Q-50 under nitrogen. UV-Vis absorption and PL spectra were measured in air with a JASCO V-670 spectrophotometer and a JASCO FP-6300 FL spectrophotometer, respectively. Tapping-mode AFM measurements were performed by a Nanoscope 8 Multimode (Veeco) instrument equipped with a E-type vertical engage scanner. TEM measurement was by Tecnai F20.Fabrication and characterization of organic solar cell
A bulk heterojunction solar cell blend of poly(3-hexylthiophene) (P3HT) and the acceptor [6,6]-phenyl-C61-butyric acid methyl ester (PCBM) was used as the photovoltaic active layer. The blend solution of P3HT (Rieke matal) and PCBM (Nano-C) with a weight ratio of (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8) was prepared in monochlorobenzene (MCB) forming a final concentration of 12 mg mL−1 and the solution was stirred overnight, after which filtering of the solution was carried out using a 0.2 μm filter. Run 1 sample's active layer used P3HT:PCBM. The other photovoltaic solar cell (runs 2 and 3) added additives (gold nanoparticles and the products by ligand exchanges of gold NP with thiol-functionalized P3HT-SH) with a P3HT
0.8) was prepared in monochlorobenzene (MCB) forming a final concentration of 12 mg mL−1 and the solution was stirred overnight, after which filtering of the solution was carried out using a 0.2 μm filter. Run 1 sample's active layer used P3HT:PCBM. The other photovoltaic solar cell (runs 2 and 3) added additives (gold nanoparticles and the products by ligand exchanges of gold NP with thiol-functionalized P3HT-SH) with a P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) gold
gold![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCBM weight ratio of 1
PCBM weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1
0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8. ITO-coated glass was cleaned in sonicated solution using acetone and isopropyl alcohol and vacuum dried at 180 °C. The cleaned ITO glass was subjected to an oxygen plasma (Plasmatic Systems Inc., PLASMA-PREEN® II-862) treatment for 20 min. A spin coating process was used to synthesise PEDOT:PSS (CLEVIOSTM PVP AI 4083) as a hole transfer layer at 2500 rpm for 40 s. The active layer consisting of P3HT:PCBM was spin-coated on top of the PEDOT:PSS at 2000 rpm for 30 s. The Al electrode (90 nm) was thermally evaporated under a vacuum of 5 × 10−5 Torr and thermally annealed under a vacuum of 10−6 Torr at 140 °C for 10 min. All of the steps involved in device fabrication were carried out. Current density–voltage (J–V) spectra were measured using a Keithley 2400 (exposing the devices to stimulated AM 1.5G, 100 mW cm−2 light).
0.8. ITO-coated glass was cleaned in sonicated solution using acetone and isopropyl alcohol and vacuum dried at 180 °C. The cleaned ITO glass was subjected to an oxygen plasma (Plasmatic Systems Inc., PLASMA-PREEN® II-862) treatment for 20 min. A spin coating process was used to synthesise PEDOT:PSS (CLEVIOSTM PVP AI 4083) as a hole transfer layer at 2500 rpm for 40 s. The active layer consisting of P3HT:PCBM was spin-coated on top of the PEDOT:PSS at 2000 rpm for 30 s. The Al electrode (90 nm) was thermally evaporated under a vacuum of 5 × 10−5 Torr and thermally annealed under a vacuum of 10−6 Torr at 140 °C for 10 min. All of the steps involved in device fabrication were carried out. Current density–voltage (J–V) spectra were measured using a Keithley 2400 (exposing the devices to stimulated AM 1.5G, 100 mW cm−2 light).
3. Results and discussion
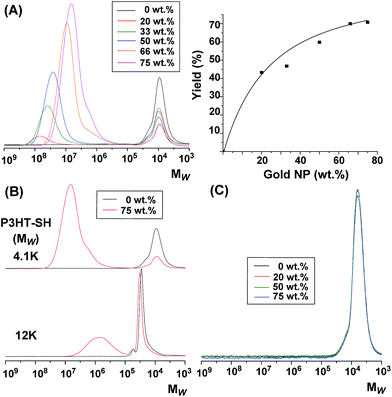
A series of well-defined P3HT-SH samples with different molecular weights were synthesized by the GRIM method, followed by end-group modifications of the obtained P3HTs, according to the literature (Scheme S1†). All synthetic steps were characterized by MALDI-TOF-MASS, SEC, 1H NMR, and FT-IR, which indicated that the thiol-functionality in the end group of P3HT was 69–82% (Fig. S1–S4†). In addition, well-defined gold NPs were prepared in the presence of HAuCl4 with oleylamine ligands as described before, which gave a particle size of 7 nm with a crystalline structure (see TEM image in Fig. 2A). Ligand exchange reactions were then carried out between these P3HT-SHs with different molecular weights and the gold NPs to examine the quantitative yield of the multi-armed star polymers with a gold NP core by changing the amount of gold NPs relative to P3HT-SH, and SEC analysis was then used to characterize the obtained materials. Fig. 1A shows the obtained SEC curves of neat P3HT-SH (Mw(MALDI) = 4.1k and PDI = 1.3) and the mixtures of P3HT-SH/gold NP with different molar ratios after 24 h at 25 °C in THF. The samples were carefully prepared to keep the concentration of P3HT-SH constant (0.25 mg mL−1 in THF) with different amounts of gold NPs, and then identical volumes were injected (100 μL) because the yields were calculated from the integration ratio of the SEC curves of P3HT-SHs. Unfortunately, SEC curves of the gold NPs were not detected both in RI and in UV detector because of scattering and absorption problems of gold NPs with oleylamine ligands. The SEC curve of P3HT-SH without gold NPs gradually and almost linearly decreased with the increase in concentration of gold NPs, while additional SEC peaks appeared at high molecular weight (Mw = 106 to 108), of which the intensities gradually increased (Fig. 1A, left). This result indicated that the linear P3HT-SHs were attached to the gold NPs by a ligand exchange reaction with oleylamine on the gold NPs to form multi-armed P3HT star polymers with a gold NP core. However, the SEC curves of P3HT-SH in the presence of gold NPs did not completely disappear, and was saturated at the ratio of P3HT-SH and gold NP (25/75 wt%) because of the limited end-functionality of P3HT-SH (82%) and the steric hindrance of the P3HT arm chains with the increased number of arm chains on the gold NP.In addition, the peak tops of the newly appeared SEC curves at high molecular weight were gradually shifted to lower molecular weights with the increase in gold NP concentration, which was probably due to the hydrodynamic volume changes of the star polymers with a different number of arm chains. This number of arm chains on gold NPs could be quantitatively calculated from the integration ratio of the SEC curves of P3HT-SHs and the density of gold NPs. For this, we assumed the deduced amounts of P3HT-SHs in the presence of gold NPs were completely attached to a gold NP, and the density of gold NPs was the same as in the bulk (19.3 g cm−3) because of the face-centered cubic (FCC) crystalline structure of gold NPs in TEM analysis (see inset of Fig. 2A). Table 1 summarizes the weight averaged molecular weight (Mw), molecular weight distribution (PDI), the yield of star polymer and the number of arm chains with different ratios of P3HT-SH to gold NPs. The absolute molecular weight and the size of the obtained star polymers were important to evaluate their hydrodynamic volumes in solution, however, these were not achieved in this study because the P3HT arm chain absorbed the laser from dynamic light scattering (DLS). As the ratio of gold NPs to P3HT-SH increased, the yield of the star polymer increased while the number of arm chains decreased. These results could be interpreted as such that a large number of star polymers with a gold NP core was formed with a small number of arm chains. In detail, the SEC peak tops of the obtained star polymers were shifted to lower molecular weights, while their intensities increased with the increase of gold NPs, because the number of star polymers was dependant on the amount of gold NPs in the limited amount of P3HT-SH, which gave a decreased number of arm chains on the gold NP. These results also corresponded well to a previous report with CdSe quantum dots (QD) and an amine functionalized polymer.42 In this case, the highest yield of star polymer (∼70%) was achieved with 66 wt% of gold NPs, which gave ∼124 arm chains on a gold NP core. However, such a high yield was not obtained with the higher molecular weight P3HT-SH (Mw(MALDI) = 12.0k and PDI = 1.3, end functionality = 69%) in the same optimized conditions (P3HT-SH/gold NP = 25/75 wt%), which was probably due to steric hindrance by the increased molecular weight (Fig. 1B). The ligand exchange reaction for non-end functionalized P3HT was also examined under the same reaction conditions with different amounts of gold NPs. This non-end functionalized P3HT was allyl group terminated P3HT (P3HT-allyl) (Mw(MALDI) = 4.2k, PDI = 1.1), which was a precursor for the synthesis of P3HT-SH (see Scheme S1†). Fig. 1C showed SEC curves of the obtained product after the reaction with P3HT-allyl and gold NPs, however, there were no significant changes in comparison with P3HT-allyl without gold NPs, and no additional SEC curves were appeared at high molecular weight. These differences between P3HT-SH and P3HT-allyl with gold NPs were also observed in solution, in terms of their long term stability. Thus, P3HT-SH/gold NP was stable after 1 week, while P3HT-allyl/gold NP aggregated and precipitated (Fig. S5†). These results indicated that the thiol group in the end of P3HT was only effective to form the star polymers with a gold NP core by a ligand exchange reaction, although P3HT possessed a lone pair of electrons on the sulfur atom in the backbone. In addition, the yield and the number of arm chains of the star polymer were strongly dependant on the ratio of gold NPs to P3HT-SH, and the molecular weight of P3HT-SH.
Fig. 2A–C shows TEM images of the gold NP precursor, and P3HT/gold NP (25/75 wt%) products from P3HT-SH and P3HT-allyl, respectively. The gold NP precursor, consisting of oleylamine ligands, showed a controlled 7 nm size with an FCC crystalline structure. The product, after ligand exchange reaction with P3HT-SH, also showed well-dispersed gold NPs with no aggregation, indicating that P3HT chains were well replaced on the gold NP to form star polymers with a gold NP core, of which the size was almost the same as the original gold NP precursor. However, those from P3HT-allyl showed severe aggregations, which were mostly located between the fibrils that originated from the P3HT-allyl chains, because highly regioregular P3HT, especially that synthesized by the GRIM method, often generates a self-organized fibril structure based on a semi-crystalline lamella morphology through intermolecular pi–pi stacking interactions. However, these fibrils were interestingly not detected in the P3HT star polymer with a gold NP core. It was already shown in our previous study with a P3HT star polymer that this is because of the unique 3-dimensional P3HT arm chains radiating from a core.13 These results were more clearly observed in AFM analysis. Fig. 3A–C shows the phase images of thin films of the linear P3HT-SH and P3HT/gold NP (25/75 wt%) products from P3HT-SH and P3HT-allyl, respectively, which were prepared on a silicon wafer by a drop casting method (1 mg mL−1 in THF). The linear P3HT-SH showed fibril structures with thicknesses of 20 nm, as expected,43 which almost disappeared, however, in the presence of gold NPs. Instead, additional embossed round shaped structures appeared and dispersed in the film, indicating the formation of the P3HT star polymer with a gold NP core. The size obtained was ca. 80 nm, which was much bigger than that of the gold NP core, which was probably due to the P3HT arm chains, as well as the flattening of the sphere shaped star polymers in solution during solidification.
 | ||
| Fig. 2 TEM images of gold NPs with oleylamine ligands (inset: electron diffraction pattern) (A) and the products by ligand exchange reaction of the gold NPs with P3HT-SH (B) and P3HT-allyl (C). | ||
In the product from P3HT-allyl, both the gold NPs and the fibril from P3HT-allyl appeared separately. This indicated that the non-functionalized P3HT-allyl was almost not interacting with the gold NPs. Interestingly, the thickness of the P3HT-allyl fibril was much thinner (10 nm) than that of the original P3HT-allyl, indicating that the gold NPs affected the crystallization of P3HT. These changing crystalline structures of P3HT in the absence or presence of gold NPs were then examined by DSC analysis (Fig. 4A). The samples were measured between 20 °C and 250 °C with a rate of change of 10 °C min−1 under N2. The melting (Tm) and the crystalline temperatures (Tc) of the linear P3HT-SH appeared at 220 °C and 186 °C, respectively, which corresponded well with conventional P3HT (Fig. 4A-a).5,44 However, those of P3HT-SH with the gold NPs completely disappeared after the ligand exchange reaction with the gold NPs (Fig. 4A-b), indicating that the P3HT star polymer was not crystallized because the tethered P3HT arm chains on the gold NP restricted the intra- and inter-chain interactions, which inhibited crystallization. However, those of P3HT-allyl with gold NPs were shifted a little to lower temperatures (Tm = 210 °C and Tc = 173 °C), and the enthalpy changes were also much smaller than those from the linear P3HT-SH, because the gold NPs within the P3HT chains disturbed the crystallization of P3HT-allyl (Fig. 4A-c). These results corresponded well to the results from TEM and AFM analyses. Fig. 4B shows the TGA results of P3HT-SH (a) and the products of P3HT-SH (b) and P3HT-allyl (c) with gold NPs (75 wt%). The samples were first held isothermally at 80 °C for 10 min, and the temperature was then increased to 800 °C at a rate of 10 °C min−1 under N2. Both P3HT-SH and the products of P3HT-SH and P3HT-allyl with gold NPs were stable until 400 °C, indicating no effect of the gold NPs on the thermal stability of P3HT-SH. P3HT-SH then decomposed to give a 72% weight loss at 650 °C, while P3HT-SH and P3HT-allyl with gold NPs gave 26% and 30% weight loss, respectively, which was mostly due to P3HT and a small amount of unexchanged oleylamine in the gold NPs. A little larger weight loss in P3HT-allyl/gold NPs indicated that P3HT-SH was effectively bonded with the gold NPs by the ligand exchange reaction.
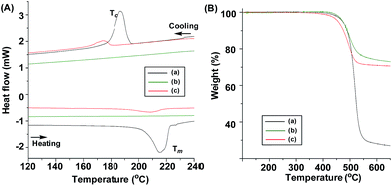 | ||
| Fig. 4 DSC (A) and TGA (B) curves of P3HT-SH (a) and the products by ligand exchange of gold NPs with P3HT-SH (b) and P3HT-allyl (c). | ||
The optical properties were then characterized using UV-Vis and PL analyses to examine the effect of gold NPs on P3HT, such as the surface plasmon resonance (SPR) of gold NPs in the presence of P3HT-SH and P3HT-allyl, both in solution and in solid states. Fig. 5A and B show UV-Vis absorptions and PL emissions of P3HT/gold NP products from P3HT-SH (a) and P3HT-allyl (b) in THF with different weight fractions of gold NPs, respectively. P3HT-SH and P3HT-allyl without the gold NPs showed identical broad absorption peaks at 445 nm, derived from the pi–pi* transition of the conjugation structures. Those monomodal shapes of P3HTs were then gradually changed to bimodal shapes with small absorption peaks at 520 nm in the presence of gold NPs due to SPR from the gold NPs (see Fig. S6†), the intensities of which gradually increased with an increase of the weight fraction of gold NPs. However, the absorption intensities from the gold NPs with P3HT-SH were much higher than those with P3HT-allyl; for example, the intensity from P3HT-SH with 66 wt% gold NPs was almost 2 times higher than that of P3HT-allyl (Fig. 5A, a and b). These results showed that SPR from the gold NPs was much enhanced when the gold NPs were in the cores of the P3HT star polymer, which was probably due to better dispersion stability in comparison to the aggregated gold NPs with P3HT-allyl chains. PL emissions of P3HT-SH and P3HT-allyl at λabs = 445 nm were observed at 565 nm, which gradually decreased with the increase of gold NPs, which was probably due to fluorescence quenching through photo-induced energy transfer from P3HT to the gold NPs or to the other P3HT (Fig. 5B, a and b). This decrease was significantly observed in the P3HT star polymer with a gold NP core, indicating that the distances from P3HT to gold NPs or to other P3HT molecules were much smaller when the P3HT arm chains were directly attached to the gold NP core in the star polymer, which was remarkable in the higher yield of star polymer. Those differences were also observed in thin film solid states, which were prepared by spin coating a THF solution (2 wt%) on a glass slide (Fig. 6). UV-Vis absorptions of P3HT-SH and P3HT-allyl without gold NPs showed broad trimodal shapes centred at 552 nm with two shoulders at 520 nm and 600 nm, derived from the pi–pi* transition with vibronic structures, respectively. These were apparently red-shifted by 107 nm in comparison to those in solution, because of the increased conjugation length through ordered pi–pi stacking of P3HT chains (Fig. 5A and 6A). Both absorption peaks were changed in the presence of gold NPs, and thus the peak tops were a little blue shifted, and a few enhanced peaks appeared at 510 nm because the gold NPs altered the conjugation length of P3HT and SPR absorption at 540 nm (see Fig. S6†). As the same in solution, SPR absorption from the gold NPs was greatly enhanced with P3HT-SH, due to its better dispersion in the thin film (see Fig. 2B and 3B). PL emissions of P3HT-SH and P3HT-allyl appeared at 650 nm after excitation at 520 nm, and those with gold NPs also appeared at the same position, indicating that the conjugation length of the P3HTs was not affected by the gold NPs. However, those intensities were significantly different in the presence of gold NPs. In comparison to the PL emissions of P3HT-SH, those with small amounts of gold NPs (∼50 wt%) were increased a little, but those with a large amount of gold NPs (75 wt%) were slightly decreased. PL emissions of P3HT-allyl were entirely enhanced in the presence of gold NPs, which was not observed in the solution. For example, the emissions of those with 33 wt% gold NPs increased by over 3 times compared to those without gold NPs. However, this was not directly proportional to the gold NPs, and thus it decreased with 50 wt% gold NPs and increased again with 75 wt% gold NPs. This interesting PL emission enhancement of P3HT-allyl with gold NPs was observed only in the solid state, which was probably due to increased intermolecular spaces by the aggregated gold NPs within the P3HT-allyl chains, which reduced the PL quenching from P3HT.45 However, it was observed a little in the P3HT star polymer with a gold NP core because P3HT arm chains were attached to the gold NPs. Consequently, the multi-armed P3HT star polymer with a gold NP core enhanced SPR absorption from the gold NPs both in solution and in solid, because of well-dispersed gold NPs by the P3HT arm chains. However, the PL emission from P3HT was diminished because of molecular contact between P3HT and the gold NPs.
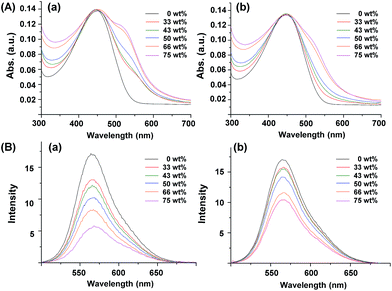 | ||
| Fig. 5 UV-Vis absorptions (A) and PL emissions (B) of P3HT-SH (a) and P3HT-allyl (b) with different weight fractions of gold NPs in THF solution. | ||
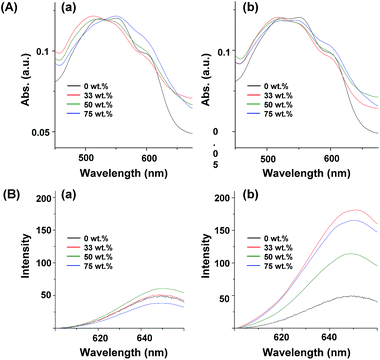 | ||
| Fig. 6 UV-Vis absorptions (A) and PL emissions (B) of P3HT-SH (a) and P3HT-allyl (b) with different weight fractions of gold NPs in a thin film on a glass slide. | ||
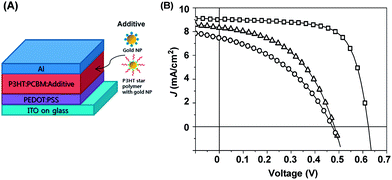
As mentioned above, gold NPs are often employed in organic solar cells to enhance optical absorption in the active layer by the SPR effect, which results in an increase of power conversion efficiency (PCE). Fig. 7A shows the schematic structure of a conventional organic solar cell consisting of ITO/PEDOT:PSS/P3HT:PCBM/Al, where gold NPs are used either on top of the ITO electrode, within the PEDOT:PSS hole transport layer or within the P3HT:PCBM active layer. Notably, gold NPs in the active layer have shown to be interesting, as light absorption could be maximized because SPR from the gold NPs occurred near the conducting polymer.31,46,47 A P3HT star polymer with a gold NP core might be a good candidate to introduce gold NPs in the active layer, because the P3HT arm chains on the gold NP are well compatible with the P3HT based active layer, which gives better dispersion of the gold NPs in the active layer. Fig. 7B shows current density (J) and voltage (V) curves of an organic solar cell with gold NPs, and a P3HT star polymer with gold NPs in the active layer of PCBM:P3HT (10 wt% gold NPs), as well as those without gold NPs (reference) for comparison. The characterized parameters obtained are also summarized in Table 2. Unexpectedly, both Jsc and Voc of the solar cells with gold NPs in the active layer were significantly decreased in comparison to those without gold NPs (Jsc = 9.01 mA cm−2 and Voc = 0.62 V). Especially those with the P3HT star polymer with gold NPs (Jsc = 7.47 mA cm−2 and Voc = 0.47 V) were a little more decreased than those with gold NPs only (Jsc = 8.33 mA cm−2 and Voc = 0.48 V). Those fill factors (FF) and PCEs were also poor in the presence of gold NPs. For example, the organic solar cell with P3HT star polymer with gold NPs showed a FF of 39.97% and a PCE of 1.41%, which were less than half of those without gold NPs (FF = 69.35% and PCE = 3.90%). These preliminary results indicated that gold NPs in the active layer was not effective, and rather, had a negative influence on the device efficiency. Perhaps the gold NPs in the active layer not only disturbed the appropriate interpenetrating structure of P3HT and PCBM, but also directly transferred excited electrons between the surface of the gold NPs and P3HT or PCBM, which was more accelerated in the P3HT star polymer with gold NPs, because of its increased compatibility with the active layer. In a forthcoming paper, we design an insulating layer between the gold NP core and the P3HT arm chain to overcome such a problem, and more systematically examine this within an organic solar cell.
4. Conclusions
The arm-first method-based ligand exchange reaction between linear end-functionalized P3HT-SH and gold NPs successfully gave a multi-armed P3HT star polymer with a gold NP core with a relatively high molecular weight (Mw = 106 to 108, PDI = ∼2.1), which was not achieved with non-functionalized P3HT-allyl. The yields of the star polymers and the number of arm chains on each gold NP were strongly dependant on the ratio of gold NPs to P3HT-SH, and the molecular weight of P3HT-SH. Gold NPs, as the cores of the star polymer, were well-dispersed without aggregation both in solution and in a thin film solid, and P3HT, as the arm chain of the star polymer, was not crystallized through pi–pi stacking interactions due to its 3-dimensional structure. In addition, surface plasmon resonance (SPR) absorption from the gold NPs was more enhanced in the P3HT star polymer both in solution and in solid, because gold NPs were dispersed by the P3HT arm chains through covalent bonding-based molecular contacts, which rather accelerated the quenching of PL emission from P3HT. The P3HT star polymer with a gold NP core was then introduced in a PCBM:P3HT active layer of the organic solar cell because of its enhanced SPR effect and compatibility with P3HT. However, the efficiency was decreased in the presence of the gold NPs, which was probably due to direct electron transfer between the surface of the gold NPs and P3HT or PCBM.Acknowledgements
This work was financially supported by the Fundamental R&D for Core Technology of Materials funded by the Ministry of Knowledge Economy, Republic of Korea and partially supported by the KIST-UNIST Partnership Program (2V04470).Notes and references
- D. Spoltore, T. Vangerven, P. Verstappen, F. Piersimoni, S. Bertho, K. Vandewal, N. V. d. Brande, M. Defour, B. V. Mele, A. D. Sio, J. Parisi, L. Lutsen, D. Vanderzande, W. Maes and J. V. Manca, Org. Electron., 2015, 21, 160 CrossRef CAS.
- G. Lu, J. Blakesley, S. Himmelberger, P. Pingel, J. Frisch, I. Lieberwirth, I. Salzmann, M. Oehzelt, R. D. Pietro, A. Salleo, N. Koch and D. Neher, Nat. Commun., 2013, 4, 1588 CrossRef PubMed.
- Z. L. Li, S. C. Yang, H. F. Meng, Y. S. Chen, Y. Z. Yang, C. H. Liu, S. F. Horng, C. S. Hsu, L. C. Chen, J. P. Hu and R. H. Lee, Appl. Phys. Lett., 2004, 84, 3558 CrossRef CAS.
- H.-H. Chou, A. Nguyen, A. Chortos, J. W. F. To, C. Lu, J. Mei, T. Kurosawa, W.-G. Bae, J. B.-H. Tok and Z. Bao, Nat. Commun., 2015, 6, 8011 CrossRef CAS PubMed.
- P.-T. Wu, H. Xin, F. S. Kim, G. Ren and S. A. Jenekhe, Macromolecules, 2009, 42, 8817 CrossRef CAS.
- M. Jeffries-El, G. Sauvé and R. D. McCullough, Adv. Mater., 2004, 16, 1017 CrossRef CAS.
- M. Jeffries-El, G. Sauvé and R. D. McCullough, Macromolecules, 2005, 38, 10346 CrossRef CAS.
- D. T. Duong, B. Walker, J. Lin, C. Kim, J. Love, B. Purushothaman, J. E. Anthony and T.-Q. Nguyen, J. Polym. Sci., Part B: Polym. Phys., 2012, 50(20), 1405 CrossRef CAS.
- K.-Y. Baek, M. Kamigaito and M. Sawamoto, J. Polym. Sci., Part A: Polym. Chem., 2002, 40(14), 2245 CrossRef CAS.
- H. Gao, K. Min and K. Matyjaszewski, Macromol. Chem. Phys., 2007, 208, 1370 CrossRef CAS.
- M. Yuan, K. Okamoto, H. A. Bronstein and C. K. Luscombe, ACS Macro Lett., 2012, 1, 392 CrossRef CAS.
- K. Y. Cho, J.-W. Choi, S.-H. Lee, S. S. Hwang and K.-Y. Baek, Polym. Chem., 2013, 4, 2400 RSC.
- H.-J. Kim, Y. J. Lee, S. S. Hwang, D. H. Choi, H. Yang and K.-Y. Baek, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 4221 CAS.
- X. Pang, C. Feng, H. Xu, W. Han, X. Xin, H. Xia, F. Qiuc and Z. Lin, Polym. Chem., 2014, 5, 2747 RSC.
- D. Han, X. Tong and Y. Zhao, J. Polym. Sci., Part A: Polym. Chem., 2012, 50(20), 4198 CrossRef CAS.
- T. Z. Oo, N. Mathews, G. Xing, B. Wu, B. Xing, L. H. Wong, T. C. Sum and S. G. Mhaisalkar, J. Phys. Chem. C, 2012, 116(10), 6453 CAS.
- L. Guo, Y. Xu, A. R. Ferhan, G. Chen and D.-H. Kim, J. Am. Chem. Soc., 2013, 135, 12338 CrossRef CAS PubMed.
- L. Vigderman and E. R. Zubarev, Adv. Drug Delivery Rev., 2013, 65, 663 CrossRef CAS PubMed.
- S. Rana, A. Bajaj, R. Mout and V. M. Rotello, Adv. Drug Delivery Rev., 2012, 64, 200 CrossRef CAS PubMed.
- G. Nystrom, M. P. Fernandez-Ronco, S. Bolisetty, M. Mazzotti and R. Mezzenga, Adv. Mater., 2016, 28, 472 CrossRef PubMed.
- Y.-M. Wang, A. D. Lackner and F. D. Toste, Acc. Chem. Res., 2014, 47(3), 889 CrossRef CAS PubMed.
- I. Ojea-Jiménez, F. M. Romero, N. G. Bastús and V. Puntes, J. Phys. Chem. C, 2010, 114, 1800 Search PubMed.
- X. Liu, M. Atwater, J. Wang, Q. Dai, J. Zou, J. P. Brennan and Q. Huo, J. Nanosci. Nanotechnol., 2007, 7(9), 3126 CrossRef CAS PubMed.
- P. M. Shem, R. Sardar and J. S. Shumaker-Parry, J. Colloid Interface Sci., 2014, 426, 107 CrossRef CAS PubMed.
- G. H. Woehrle, L. O. Brown and J. E. Hutchison, J. Am. Chem. Soc., 2005, 127(7), 2172 CrossRef CAS PubMed.
- C.-W. Hu, Y. Huang and R. C.-C. Tsiang, J. Nanosci. Nanotechnol., 2009, 9, 3084 CrossRef CAS PubMed.
- Y. Leroux, E. Eang, C. Fave, G. Trippe and J. C. Lacroix, Electrochem. Commun., 2007, 9, 1258 CrossRef CAS.
- R. Pacios, R. Marcilla, C. Pozo-Gonzalo, J. A. Pomposo, H. Grande, J. Aizpurua and D. Mecerreyes, J. Nanosci. Nanotechnol., 2007, 7, 1 CrossRef.
- M. L. Brongersma, N. J. Halas and P. Nordlander, Nat. Nanotechnol., 2015, 10, 25 CrossRef CAS PubMed.
- A. Baba, M.-K. Park, R. C. Advincula and W. Knoll, Langmuir, 2002, 18, 4648 CrossRef CAS.
- V. Jankovic, Y. Michael Yang, J. You, L. Dou, Y. Liu, P. Cheung, Jane P. Chang and Y. Yang, ACS Nano, 2013, 7(5), 3815 CrossRef CAS PubMed.
- S. H. Kim, T.-S. Bae, W. Heo, T. Joo, K.-D. Song, H.-G. Park and S. y. Ryu, ACS Appl. Mater. Interfaces, 2015, 7(27), 15031 CAS.
- J. N. d. Freitas, M. A. Mamo, M. Maubane, W. A. L. v. Otterlo, N. J. Coville and A. F. Nogueira, J. Power Sources, 2012, 215, 99 CrossRef.
- M. Lee, B. W. Kim, J. D. Nam, Y. Lee, Y. Son and S. J. Seo, Mol. Cryst. Liq. Cryst., 2003, 407, 397 CAS.
- L. Zhai and R. D. McCullough, J. Mater. Chem., 2004, 14, 141 RSC.
- C.-H. Lai, I.-C. Wu, C.-C. Kang, J.-F. Lee, M.-L. Hoac and P.-T. Chou, Chem. Commun., 2009, 1996 RSC.
- D. Lee and D.-J. Jang, Polymer, 2014, 55, 5469 CrossRef CAS.
- P. E. Williams, S. T. Jones, Z. Walsh, E. A. Appel, E. K. Abo-Hamed and O. A. Scherman, ACS Macro Letters, 2015, 4, 255 CrossRef CAS.
- F. Monnaie, W. Brullot, T. Verbiest, J. D. Winter, P. Gerbaux, A. Smeets and G. Koeckelberghs, Macromolecules, 2013, 46(21), 8500 CrossRef CAS.
- K. Palaniappan, J. W. Murphy, N. Khanam, J. Horvath, H. Alshareef, M. Quevedo-Lopez, M. C. Biewer, S. Y. Park, M. J. Kim, B. E. Gnade and M. C. Stefan, Macromolecules, 2009, 42, 3845 CrossRef CAS.
- M. Park, B. D. Chin, J.-W. Yu, M.-S. Chun and S.-H. Han, J. Ind. Eng. Chem., 2008, 14, 382 CrossRef CAS.
- M. Wang, T. E. Dykstra, X. Lou, M. R. Salvador, G. D. Scholes and M. A. Winnik, Angew. Chem., Int. Ed., 2006, 45, 2221 CrossRef CAS PubMed.
- M. Brinkmann, J. Polym. Sci., Part B: Polym. Phys., 2011, 49, 1218 CrossRef CAS.
- O. F. Pascui, R. Lohwasser, M. Sommer, M. Thelakkat, T. T. Albrecht and K. Saalwachter, Macromolecules, 2010, 43, 9401 CrossRef CAS.
- P. G. Nicholson, V. Ruiz, J. V. Macpherson and P. R. Unwin, Chem. Commun., 2005, 1052 RSC.
- A. Y. Mahmoud, J. Zhang, D. Ma, R. Izquierdo and V.-V. Truong, Sol. Energy Mater. Sol. Cells, 2013, 116, 1 CrossRef CAS.
- W. Shen, J. Tang, R. Yang, H. Cong, X. Bao, Y. Wang, X. Wang, Z. Huang, J. Liu, L. Huang, J. Jiao, Q. Xu, W. Chen and L. A. Belfiore, RSC Adv., 2014, 4, 4379 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: MALDI-TOF-MASS, SEC, 1H NMR, and FT-IR of P3HT-SH and P3HT-allyl. See DOI: 10.1039/c6ra06926f |
| This journal is © The Royal Society of Chemistry 2016 |