Synthesis of (E)-oxindolylidene acetate using tandem palladium-catalyzed Heck and alkoxycarbonylation reactions†
Wei-Jen
Lin
a,
Kak-Shan
Shia
b,
Jen-Shin
Song
b,
Ming-Hsien
Wu
b and
Wen-Tai
Li
*a
aNational Research Institute of Chinese Medicine, Ministry of Health and Welfare, Taipei 11221, Taiwan. E-mail: wtli@nricm.edu.tw; Fax: +886 2 2825 0743; Tel: +886 2 2820 1999 ext. 8261
bInstitute of Biotechnology and Pharmaceutical Research, National Health Research Institutes, Miaoli 35053, Taiwan
First published on 2nd November 2015
Abstract
Tandem reactions use consecutive reaction steps to efficiently synthesize compounds of high molecular complexity. This paper presents a tandem Pd-catalyzed Heck and alkoxycarbonylation reaction for the stereoselective synthesis of (E)-oxindolylidene acetates. The mechanism underlying the Pd-catalyzed tandem reaction involves the syn-carbopalladation of ynamides followed by alkoxycarbonylation with CO and alcohol. This method makes it possible to obtain the desired (E)-configuration of oxindolylidene acetates exclusively. We evaluated the scope of the reaction by applying optimal reaction conditions to the facile synthesis of a library of (E)-oxindolylidene acetates. The resulting (E)-oxindolylidene acetates exhibited potent anticancer activities against a variety of human cancer cell lines. The anticancer activities of some (E)-oxindolylidene acetates were even superior to those of known CDK inhibitors indirubin-3′-oxime and roscovitine.
Introduction
The 3-ylideneoxindole moiety is a skeleton of pharmacological importance, which appears as the core structure in a variety of bioactive molecules exhibiting potent antifungal,1 anticancer,2 and antiviral activities.3 For example, 3-ylideneoxindole is the core structure in biologically active compounds such as sunitinib and hesperadin, which possess the capacity to bind receptor tyrosine kinase (RTK) and Aurora B with high affinity.4–6 3-Ylideneoxindole also exists in a number of natural indole alkaloids (including neolaugerine,7 costinone A, and costinine B8) which possess a variety of biological properties.Tandem reactions are a series of consecutive reactions that provide a highly efficient means of assembling compounds.9 The molecular complexity and diversity that can be created using this facile approach is suitable for combinatorial library synthesis as it is able to produce a large number of compounds using a minimum number of steps.10
The synthesis of a 3-ylideneoxindole moiety has attracted considerable interest among researchers, and impressive advances have already been made in which 3-alkylideneoxindole was used as an important intermediate such as the synthesis of TMC-95A.11 The stereoselective synthesis of various substituted 3-alkylideneoxindoles from a series of ynamides and boronic acids by way of palladium-catalyzed Heck–Suzuki–Miyaura domino reactions has also been reported.12 The domino reaction, which further expands the utility of ynamides, has been applied in the preparation of natural, biologically active products.
Oxindolylidene acetate is commonly used as a synthetic target due to its interesting biological activities.13 Previous successes involving the use of metal-catalyzed tandem reactions led us to consider whether Pd-catalyzed tandem Heck and alkoxycarbonylation reactions of ynamides with carbon monoxide (CO) and alcohols in a single pot could be used to produce oxindolylidene acetate. In the following, we report a concise method for the stereoselective synthesis of (E)-oxindolylidene acetates using the tandem Pd-catalyzed Heck and alkoxycarbonylation reactions of N-substituted propiolamides. The resulting (E)-oxindolylidene acetates exhibited potent anticancer activities against a variety of human cancer cell lines.
Results and discussion
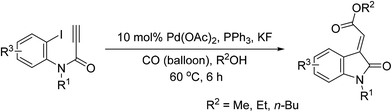
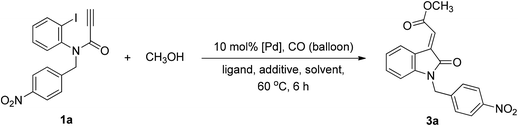
Tandem Pd-catalyzed Heck and alkoxycarbonylation reactions were combined to develop an efficient stereocontrolled method for the synthesis of (E)-oxindolylidene acetates using CO and alcohols in one pot (Chart 1). Tandem Pd-catalyzed Heck–Suzuki–Miyaura domino reactions have previously been used to synthesize 3-alkylideneoxindoles;12 however, this is the first study to report on the use of tandem metal-catalyzed reactions in the synthesis of oxindolylidene acetates from ynamides. At the outset of our investigation, N-(2-iodophenyl)-N-(4-nitrobenzyl)propiolamide (1a) was selected as the model compound for the evaluation of tandem reactions. We initially evaluated the reaction of N-(2-iodophenyl)-N-(4-nitrobenzyl)propiolamide (1a) and methanol (3 equiv.) under CO at atmospheric pressure and elevated temperatures. The reaction was conducted in the presence of triphenylphosphine (0.5 equiv.) in THF, and Pd(OAc)2 was used as a catalyst.14 The desired (E)-oxindolylidene acetate (3a) was obtained at a yield of 23% wherein only the (E)-configuration was found (Table 1, entry 1).| Entry | [Pd] | Ligand | Additive | Solvent | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1.0 equiv. of ynamide 1a (0.05 M in solvent), 3.0 equiv. of CH3OH (except entry 22), 0.1 equiv. of the Pd catalyst, 0.2 equiv. of the ligand, and 3.0 equiv. of the additive for 6 h; temperature was 60 °C and CO pressure was 1 atmosphere. b Yields refer to isolated and purified compounds. | |||||
| 1 | Pd(OAc)2 | PPh3 | — | THF | 23 |
| 2 | Pd(OAc)2 | PPh3 | K2CO3 | THF | 12 |
| 3 | Pd(OAc)2 | PPh3 | Cs2CO3 | THF | 34 |
| 4 | Pd(OAc)2 | PPh3 | KF | THF | 66 |
| 5 | Pd(OAc)2 | PPh3 | CsF | THF | 64 |
| 6 | Pd(OAc)2 | PPh3 | KI | THF | 51 |
| 7 | Pd(OAc)2 | — | KF | THF | 14 |
| 8 | Pd(OAc)2 | dppp | KF | THF | 15 |
| 9 | Pd(OAc)2 | dppe | KF | THF | 24 |
| 10 | Pd(OAc)2 | dppe | KI | THF | 18 |
| 11 | Pd(OAc)2 | PPh2Me | KF | THF | 41 |
| 12 | Pd(OAc)2 | PCy3 | KF | THF | 21 |
| 13 | Pd(PPh3)4 | — | KF | THF | 38 |
| 14 | Pd(PPh3)2Cl2 | — | KF | THF | 43 |
| 15 | Pd2(dba)3 | PPh3 | KF | THF | 36 |
| 16 | Pd(dppf)Cl2 | — | KF | THF | 65 |
| 17 | Pd(OAc)2 | PPh3 | KF | m-Xylene | 19 |
| 18 | Pd(OAc)2 | PPh3 | KF | CH3CN | 8 |
| 19 | Pd(OAc)2 | PPh3 | KF | DME | 47 |
| 20 | Pd(OAc)2 | PPh3 | KF | 1,4-Dioxane | 22 |
| 21 | Pd(OAc)2 | PPh3 | KF | DMSO | 5 |
| 22 | Pd(OAc)2 | PPh3 | KF | CH3OH | 71 |
Notably, additives and phosphine ligands were shown to play important roles in the Pd-catalyzed tandem reaction. In fact, the addition of KF increased the yield from 23% to 66% (Table 1, entry 4); therefore, we subsequently turned our attention to the evaluation of various additives with the aim of further improving the reaction yields (Table 1, entries 2–6). A number of carbonates, such as potassium carbonate and cesium carbonate, were used as additives; however, the resulting yields were lower than the yield achieved using KF (Table 1, entries 2–4). Conversely, using potassium fluoride, cesium fluoride, or potassium iodide as additives resulted in better yields of (E)-oxindolylidene acetate (Table 1, entries 4–6). Phosphine ligands were also shown to exert a strong effect on the yield of the Pd-catalyzed tandem reaction, as the reaction without the phosphine ligand resulted in poor yield (Table 1, entry 7), and the reactions with bulky bidentate ligands (dppp and dppe) or monodentate phosphine ligands (PPh2Me and PCy3), provided only slightly better yields than reactions without the addition of ligands (Table 1, entries 8–12). As shown in Table 1, PPh3 proved to be a more effective ligand for this reaction and others. A variety of palladium catalysts were subsequently used to evaluate the stereoselective synthesis of (E)-oxindolylidene acetate under CO and atmospheric pressure at elevated temperatures. The palladium catalysts Pd(OAc)2 and Pd(dppf)Cl2 proved efficient for this tandem reaction; however, other palladium catalysts produced lower yields (Table 1, entries 4 and 13–16).
We further evaluated how a variety of solvents affected this Pd-catalyzed tandem reaction. Among these, methanol and THF provided the best results. In addition, trace quantities of N-substituted dimethyl 2-(2-oxoindolin-3-ylidene)malonate was also observed in this reaction, perhaps due to the presence of trace quantities of oxygen, which may have caused the oxidative alkoxycarbonylation of the terminal alkyne in the first step.15
After establishing the optimal reaction conditions, we investigated a series of N-substituted propiolamides 1 with various primary, secondary, and aryl alcohols in order to determine the scope of the reaction (Table 2). Alkoxycarbonylation is sensitive to the steric features of alcohols, and we observed that when N-substituted propiolamides 1 and various primary aliphatic alcohols, such as methanol, ethanol, and n-butanol underwent Pd-catalyzed tandem Heck and alkoxycarbonylation reactions the yield was good. Conversely, isopropanol, phenol, and benzyl alcohols were unreactive (Table 2, entries 34–36). In addition, The Pd-catalyzed tandem Heck-alkoxycarbonylation reactions revealed excellent functional group tolerance with benzyl substituents at R1 bearing electron withdrawing groups at the phenyl ring, such as nitro, methyl ester, trifluoromethyl and cyano resulting in a smooth reaction, resulting in corresponding oxindolylidene acetates 3a, 3f, 3g and 3h in good yields. Benzyl and p-methoxybenzyl in the R1 position were also well tolerated in the reaction, thereby producing corresponding oxindolylidene acetates 3m and 3b with yields of 64% and 67%, respectively. In addition, substitution at the R1 position with an isoxazolylmethyl ring, methyl and H, the products 3n, 3t and 3u were obtained exclusively in fair yields. Finally, the reaction conditions were found to be favorable when functional groups 5-methyl, 5-methoxyl, and 5-chloride were substituted at R3 (Table 2, entries 17, 18, 27 and 28), such that the resulting reactions respectively produced corresponding oxindolylidene acetates 3q, 3r, 4e and 4f in good yields.
| Entry | R1 | R2 | R3 | Product | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1.0 equiv. of ynamides 1 (0.05 M in CH3OH), 0.1 equiv. of the Pd catalyst, 0.2 equiv. of PPh3 and 3.0 equiv. of KF for 6 h; temperature was 60 °C and CO pressure was 1 atmosphere. b Yields refer to isolated and purified compounds, and only the (E)-configuration of oxindolylidene acetate was found. c The yield was negligible. | |||||
| 1 | p-NO2-PhCH2 | Me | H | 3a | 71 |
| 2 | p-MeO-PhCH2 | Me | H | 3b | 67 |
| 3 | p-F-PhCH2 | Me | H | 3c | 60 |
| 4 | p-Cl-PhCH2 | Me | H | 3d | 68 |
| 5 | p-Br-PhCH2 | Me | H | 3e | 64 |
| 6 | p-CO2Me-PhCH2 | Me | H | 3f | 74 |
| 7 | p-CF3-PhCH2 | Me | H | 3g | 70 |
| 8 | p-CN-PhCH2 | Me | H | 3h | 66 |
| 9 | m-CN-PhCH2 | Me | H | 3i | 51 |
| 10 | m-Cl-PhCH2 | Me | H | 3j | 58 |
| 11 | 2-Np-CH2 | Me | H | 3k | 59 |
| 12 | o-Cl-PhCH2 | Me | H | 3l | 57 |
| 13 | Bn | Me | H | 3m | 64 |
| 14 | 3-Methyl-5-isoxazolylmethyl | Me | H | 3n | 55 |
| 15 | 2,6-Cl2-PhCH2 | Me | H | 3o | 59 |
| 16 | 3,4,5-(MeO)3-PhCH2 | Me | H | 3p | 68 |
| 17 | p-Cl-PhCH2 | Me | 5-Me | 3q | 67 |
| 18 | p-Cl-PhCH2 | Me | 5-OMe | 3r | 64 |
| 19 | (6-Bromobenzo[d][1,3]dioxol-5-yl)CH2 | Me | H | 3s | 54 |
| 20 | Me | Me | H | 3t | 61 |
| 21 | H | Me | H | 3u | 51 |
| 22 | p-NO2-PhCH2 | Me | 5-CF3 | 3v | 45 |
| 23 | p-NO2-PhCH2 | Et | H | 4a | 62 |
| 24 | p-Cl-PhCH2 | Et | H | 4b | 59 |
| 25 | p-Br-PhCH2 | Et | H | 4c | 57 |
| 26 | 3,4,5-(MeO)3-PhCH2 | Et | H | 4d | 64 |
| 27 | p-Cl-PhCH2 | Et | 5-Cl | 4e | 55 |
| 28 | p-Cl-PhCH2 | Et | 5-OMe | 4f | 61 |
| 29 | Me | Et | H | 4g | 58 |
| 30 | m-CN-PhCH2 | n Bu | H | 5a | 57 |
| 31 | p-CN-PhCH2 | n Bu | H | 5b | 53 |
| 32 | p-NO2-PhCH2 | nBu | H | 5c | 55 |
| 33 | H | Et | H | 4h | 58 |
| 34 | p-NO2-PhCH2 | iPr | H | —c | |
| 35 | p-NO2-PhCH2 | Ph | H | —c | |
| 36 | p-NO2-PhCH2 | Bn | H | —c | |
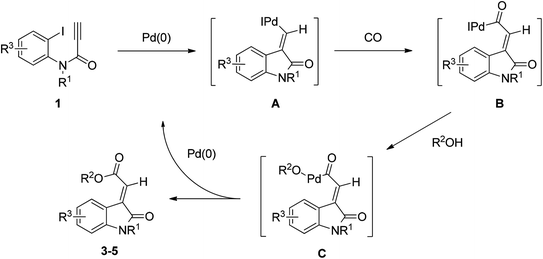
One plausible mechanism for the formation of compounds 3–5 is presented in Scheme 1. The formation of the products may initially have followed the Heck reaction mechanism, which involves the syn-carbopalladation of ynamide with a palladium catalyst to form the intermediate A. The resulting products 3–5 were obtained following alkoxycarbonylation of intermediate A treated with CO and alcohols. This mechanism involves a syn migratory insertion of the alkyne into the Ar–Pd bond, followed by the alkoxycarbonylation of CO and alcohols, leading to the only (E)-configuration of oxindolylidene acetate.
All of the synthesized (E)-oxindolylidene acetates 3–5 obtained via tandem Pd-catalyzed reactions were evaluated for anticancer activities using human NCI-H460 lung cancer cells. We then studied the structure–activity relationships (SARs) in compounds containing various substitutions in the (E)-oxindolylidene acetate scaffold. Specifically, we examined the antiproliferative effects of various R1 and R2 groups on oxindolylidene acetates. Compounds 3d, 3h, and 5b, containing para-substituents on the phenyl ring of the R1 group, presented slightly better anticancer activity than did compounds 3i, 3j, 3l, and 5a, which contained meta- and ortho-substituents on the phenyl ring of oxindolylidene acetates. Interestingly, compound 3n, containing an isoxazolyl ring, was found to decrease the anticancer activity to below that of compounds with a phenyl ring at R1. In our evaluation of the anticancer activities of methyl, ethyl, and n-butyl groups at R2, the activity of the methyl group was found to be superior to that of ethyl and n-butyl groups, and the order of activity of R2 groups against NCI-H460 cancer cells can be described as methyl > ethyl > n-butyl in most examples. However, substitutions at R3 did not have significant effects on anticancer activity (Table 3).
| Compound | IC50 (μM) | Compound | IC50 (μM) |
|---|---|---|---|
| a IC50 values represent the mean ± SD of three determinations. b Reported IC50 = 13.1 μM. | |||
| 3a | 5.2 ± 0.7 | 3r | 3.7 ± 0.1 |
| 3b | 5.1 ± 0.4 | 3s | 18.8 ± 1.9 |
| 3c | 6.3 ± 0.3 | 3t | 7.8 ± 1.6 |
| 3d | 6.8 ± 0.8 | 3u | 5.5 ± 0.4 |
| 3e | 7.4 ± 0.6 | 4a | 8.7 ± 0.5 |
| 3f | 4.3 ± 0.3 | 4b | 6.8 ± 0.7 |
| 3g | 2.0 ± 0.1 | 4c | 8.3 ± 0.5 |
| 3h | 5.3 ± 0.6 | 4d | 6.2 ± 0.3 |
| 3i | 8.9 ± 0.7 | 4e | 9.3 ± 0.7 |
| 3j | 9.6 ± 0.5 | 4f | 7.7 ± 0.8 |
| 3k | 4.2 ± 0.8 | 4g | 11.8 ± 1.3 |
| 3l | 6.2 ± 0.6 | 5a | 17.3 ± 1.6 |
| 3m | 5.1 ± 0.7 | 5b | 14.9 ± 1.9 |
| 3n | 52.0 ± 3.4 | 5c | 19.2 ± 1.7 |
| 3o | 4.4 ± 1.5 | 4h | 38.6 ± 3.7 |
| 3p | 1.2 ± 0.1 | Indirubin-3′-oxime | 32.6 ± 5.1 |
| 3q | 3.9 ± 0.4 | Roscovitine | 11.6 ± 1.9b |
Further evaluation of anticancer activities was conducted on selected active (E)-oxindolylidene acetates using six different cancer cell lines: human lung adenocarcinoma cell CL 1–5, human epidermoid carcinoma KB, human gastric cancer MKN-45, human breast adenocarcinoma MCF-7, human pancreatic carcinoma MIA Paca-2, and human central nervous system cancer SF-268 (Table 4). Most of the 3-ylideneoxindole acetamides exhibited a broad spectrum of anticancer activity against human cancer cells, with effects even more pronounced than those associated with well-known anticancer drug candidates indirubin-3′-oxime and roscovitine.
| Compd | CL 1–5 | KB | MKN-45 | MCF-7 | MIA Paca-2 | SF-268 |
|---|---|---|---|---|---|---|
| a IC50 values represent the mean ± SD of three determinations. b Reported IC50 = 30.1 μM. c Reported IC50 = 14.7 μM. | ||||||
| 3b | 4.6 ± 0.5 | 5.5 ± 1.7 | 3.4 ± 0.6 | 27.1 ± 0.5 | 39.4 ± 2.8 | 3.7 ± 0.4 |
| 3f | 5.7 ± 0.6 | 5.6 ± 0.6 | 5.0 ± 0.6 | 8.5 ± 5.1 | 38.3 ± 2.2 | 3.9 ± 0.5 |
| 3g | 5.3 ± 0.2 | 4.3 ± 0.3 | 5.1 ± 0.2 | 8.3 ± 4.5 | 37.6 ± 2.8 | 4.4 ± 0.6 |
| 3h | 7.3 ± 0.3 | 4.9 ± 0.1 | 6.4 ± 0.1 | 21.4 ± 1.3 | 35.0 ± 2.5 | 15.9 ± 1.4 |
| 3k | 5.2 ± 0.2 | 11.0 ± 3.4 | 4.5 ± 0.4 | 23.9 ± 0.3 | 39.1 ± 3.3 | 5.8 ± 0.4 |
| 3o | 6.1 ± 0.5 | 5.3 ± 0.3 | 6.6 ± 0.3 | 17.5 ± 1.5 | 37.1 ± 2.1 | 5.6 ± 0.5 |
| 3p | 3.7 ± 0.2 | 4.5 ± 0.2 | 3.2 ± 0.2 | 5.6 ± 1.4 | 30.2 ± 1.7 | 3.4 ± 0.3 |
| 3q | 7.0 ± 0.6 | 11.9 ± 3.2 | 12.2 ± 4.1 | 25.2 ± 3.5 | 37.3 ± 10.7 | 12.4 ± 2.6 |
| 3r | 3.1 ± 0.2 | 3.5 ± 0.4 | 3.6 ± 0.6 | 4.6 ± 0.9 | 38.6 ± 2.4 | 5.4 ± 0.3 |
| Indirubin-3′-oxime | 21.2 ± 1.3 | 19.2 ± 0.6 | 36.7 ± 2.0 | 25.5 ± 3.2 | 29.5 ± 3.7 | 37.5 ± 3.8 |
| Roscovitine | 8.9 ± 0.1 | 24.6 ± 1.9b | 7.6 ± 2.2 | 19.6 ± 2.2c | 17.9 ± 0.8 | 11.6 ± 1.5 |
In summary, this study developed tandem Pd-catalyzed Heck and alkoxycarbonylation reactions for the production of new anticancer agents based on (E)-oxindolylidene acetates. This stereoselective reaction proceeds through the key steps of syn-carbopalladation and alkoxycarbonylation in the presence of CO and alcohols, resulting in only the (E)-configuration of oxindolylidene acetates. We also established the optimal conditions and scope of the reactions. The resulting (E)-oxindolylidene acetates exhibit potent anticancer activity against a variety of human cancer cells.
Experimental section
General procedure for synthesis of N-(2-iodophenyl)-N-(4-nitrobenzyl) propiolamide (1a)
N-(2-Iodophenyl)propiolamide was synthesized with slight modifications according to the reported method.16 To a solution of N-(2-iodophenyl)propiolamide (400 mg, 1.48 mmol) in DMF (20 mL) was added NaHCO3 (248 mg, 2.95 mmol) portionwise. The mixture was stirred at 0 °C for 30 min and 4-nitrobenzyl chloride (329 mg, 1.92 mmol) was added dropwise. The reaction mixture was heated to reflux for 6 h until complete by TLC. The reaction was quenched by addition of water and extracted with ethyl acetate. The combined organic layers were washed with brine solution, dried over MgSO4 and concentrated in vacuo. The residue purified by flash column chromatography furnished the desired N-(2-iodophenyl)-N-(4-nitrobenzyl)propiolamide 1a (463 mg, 77%) as a yellow solid (two rotamers at a ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Mp = 145–147 °C. IR (KBr) νmax: 3210, 2106, 1646, 1520, 1345, 1310 cm−1. 1H NMR (600 MHz, CDCl3) major/minor δ: 8.13/8.16 (d, J = 8.4 Hz, 2H), 7.93/7.89 (dd, J = 7.9, 1.2 Hz, 1H), 7.40/7.42 (d, J = 8.4 Hz, 2H), 7.25/7.21 (dt, J = 7.8, 1.2 Hz, 1H), 7.08/7.01 (dt, J = 7.8, 1.2 Hz, 1H), 6.81/6.71 (dd, J = 7.8, 1.2 Hz, 1H), 5.56/5.58 (d, J = 15.0, 1H), 4.24/4.70 (d, J = 15.0 Hz, 1H), 2.78/3.35 (s, 1H). 13C NMR (150 MHz, CDCl3) major/minor δ: 153.3/152.6, 147.6/147.8, 143.0/142.7, 142.5/141.0, 140.3/140.3, 130.9/129.8, 130.7/129.6, 130.3/130.2, 129.2/129.3, 123.8/123.9, 100.0/98.1, 80.1/80.7, 75.6/75.8, 50.8/54.4. HRMS calcd for C16H12IN2O3 (M+1)+ 406.9893, found 406.9894.
1). Mp = 145–147 °C. IR (KBr) νmax: 3210, 2106, 1646, 1520, 1345, 1310 cm−1. 1H NMR (600 MHz, CDCl3) major/minor δ: 8.13/8.16 (d, J = 8.4 Hz, 2H), 7.93/7.89 (dd, J = 7.9, 1.2 Hz, 1H), 7.40/7.42 (d, J = 8.4 Hz, 2H), 7.25/7.21 (dt, J = 7.8, 1.2 Hz, 1H), 7.08/7.01 (dt, J = 7.8, 1.2 Hz, 1H), 6.81/6.71 (dd, J = 7.8, 1.2 Hz, 1H), 5.56/5.58 (d, J = 15.0, 1H), 4.24/4.70 (d, J = 15.0 Hz, 1H), 2.78/3.35 (s, 1H). 13C NMR (150 MHz, CDCl3) major/minor δ: 153.3/152.6, 147.6/147.8, 143.0/142.7, 142.5/141.0, 140.3/140.3, 130.9/129.8, 130.7/129.6, 130.3/130.2, 129.2/129.3, 123.8/123.9, 100.0/98.1, 80.1/80.7, 75.6/75.8, 50.8/54.4. HRMS calcd for C16H12IN2O3 (M+1)+ 406.9893, found 406.9894.
General procedure for synthesis of (E)-methyl 2-(1-(4-nitrobenzyl)-2-oxoindolin-3-ylidene)acetate (3a)
N-(2-Iodophenyl)-N-(4-nitrobenzyl)propiolamide 1a (200 mg, 0.49 mmol) in CH3OH (10 mL) was added Pd(OAc)2 (11 mg, 0.05 mmol), PPh3 (26 mg, 0.10 mmol) and potassium fluoride (85 mg, 1.47 mmol) in a 25 mL round flask. The flask was evacuated under vacuum and back-filled with carbon monoxide from a balloon. The cycle was repeated three times and the vessel was left open to a dynamic atmosphere of CO. The mixture was then heated to 60 °C under vigorous stirring until complete by TLC. Then the mixture was filtered and concentrated in vacuo. The residue was dissolved in EtOAc and then washed with water. The water phase was extracted with EtOAc (10 mL) twice. The organic layers were combined, dried over MgSO4 and concentrated. The crude mixture was purified by flash column chromatography to afford the title compound 3a (118 mg, 71%) as a yellowish solid. Mp = 172–174 °C. IR (KBr) νmax: 3415, 1700, 1602, 1515, 1468, 1342, 1214 cm−1. 1H NMR (600 MHz, CDCl3) δ: 8.58 (d, J = 7.8 Hz, 1H), 8.17 (d, J = 8.4 Hz, 2H), 7.43 (d, J = 8.4 Hz, 2H), 7.26 (t, J = 7.2 Hz, 1H), 7.07 (t, J = 7.8 Hz, 1H), 6.98 (s, 1H), 6.59 (d, J = 8.4 Hz, 1H), 5.02 (s, 2H), 3.88 (s, 3H). 13C NMR (150 MHz, CDCl3) δ: 167.7, 165.9, 147.6, 144.4, 142.8, 137.4, 132.6, 129.2, 128.0, 124.2, 123.4, 122.9, 120.0, 108.7, 52.3, 43.3. HRMS calcd for C18H14N2O5 (M)+ 338.0882, found 338.0892.Cancer cell growth inhibition assay
Human cancer cell lines, including human lung adenocarcinoma cell CL 1–5, human epidermoid carcinoma KB, human gastric cancer MKN-45, human breast adenocarcinoma MCF-7, human pancreatic carcinoma MIA Paca-2 and human central nervous system cancer SF-268, were seeded in 96-well plates and incubated for 24 h at 37 °C in a 5% CO2 incubator. Test compounds were dissolved in dimethylsulfoxide (DMSO) and diluted for cell treatment in culture medium containing DMSO at the final concentration of 0.5%. Cells were treated with test compounds of various concentrations in triplicates per concentration in the culture medium and incubated at 37 °C for 72 h. Actinomycin D of 10 nM and 0.3% DMSO were used as the positive and vehicle controls, respectively. A colorimetric assay using the MTS/PMS system was used to determine the cytotoxic activity of the test compounds. The optical density (OD) values at 490 nm were measured with a 1420-multilabel counter VICTOR from Wallac (Turku, Finland). The IC50, the concentration that inhibited 50% of the cancer cell growth activity, was then determined. All experiments were repeated three times.17Acknowledgements
We gratefully acknowledge financial support of the National Science Council (Grant NSC 102-2113-M-077-002-MY2).Notes and references
- (a) M. S. C. Pedras, J. L. Sorensen, F. J. Okanga and I. L. Zaharia, Bioorg. Med. Chem. Lett., 1999, 9, 3015 CrossRef CAS PubMed; (b) M. S. C. Pedras, P. B. Chumala and M. Suchy, Phytochemistry, 2003, 64, 949 CrossRef CAS PubMed.
- (a) C.-T. Chiou, W.-C. Lee, J.-H. Liao, J.-J. Cheng, L.-C. Lin, C.-Y. Chen, J.-S. Song, M.-H. Wu, K.-S. Shia and W.-T. Li, Eur. J. Med. Chem., 2015, 98, 1 CrossRef CAS PubMed; (b) A. Brancale and R. Silvestri, Med. Res. Rev., 2007, 27, 209 CrossRef CAS PubMed; (c) N. Gao, L. Kramer, M. Rahmani, P. Dent and S. Grant, Mol. Pharmacol., 2006, 70, 645 CrossRef CAS PubMed; (d) Y. Luo, F. Xiao, S. J. Qian, Q. J. He, W. Lu and B. Yang, MedChemComm, 2011, 2, 1054 RSC; (e) H. B. Zou, L. Zhang, J. F. Ouyang, M. A. Giulianotti and Y. P. Yu, Eur. J. Med. Chem., 2011, 46, 5970 CrossRef CAS PubMed.
- T. Jiang, K. L. Kuhen, K. Wolff, H. Yin, K. Bieza, J. Caldwell, B. Bursulaya, T. Tuntlad, K. Zhang, D. Karanewsky and Y. He, Bioorg. Med. Chem. Lett., 2006, 16, 2109 CrossRef CAS PubMed.
- T. M. Sielecki, J. F. Boylan, P. A. Benfield and G. L. Trainor, J. Med. Chem., 2000, 43, 1 CrossRef CAS PubMed.
- B. B. Hasinoff, D. Patel and K. A. O′Hara, Mol. Pharmacol., 2008, 74, 1722 CrossRef CAS PubMed.
- A. A. Dar, L. W. Goff, S. Majid, J. Berlin and W. El-Rifai, Mol. Cancer Ther., 2010, 9, 268 CrossRef CAS PubMed.
- B. Weniger, Y. Jiang, R. Anton, J. Bastida, T. Varea and J.-C. Quirion, Phytochemistry, 1993, 32, 1587 CrossRef CAS.
- I. Fatima, I. Ahmad, S. A. Nawaz, A. Malik, N. Afza, G. Luttfullah and M. I. Choudhary, Heterocycles, 2006, 68, 1421 CrossRef CAS.
- A. Shaabani, A. Maleki, H. Mofakham and H. R. Khavasi, J. Comb. Chem., 2008, 10, 883 CrossRef CAS PubMed.
- (a) I. Akritopoulou-Zanze, Curr. Opin. Chem. Biol., 2008, 12, 324 CrossRef CAS PubMed; (b) P. Janvier, X. Sun, H. Bienaymé and J. Zhu, J. Am. Chem. Soc., 2002, 124, 2560 CrossRef CAS PubMed.
- (a) S. Lin, Z. Q. Yang, B. H. Kwok, M. Koldobskiy, C. M. Crews and S. J. Danishefsky, J. Am. Chem. Soc., 2004, 126, 6347 CrossRef CAS PubMed; (b) S. Lin and S. J. Danishefsky, Angew. Chem., Int. Ed., 2002, 41, 512 CrossRef CAS; (c) M. Inoue, H. Furuyama, H. Sakazaki and H. Hirama, Org. Lett., 2001, 3, 2863 CrossRef CAS PubMed.
- (a) R. Yanada, S. Obika, M. Oyama and Y. Takemoto, Org. Lett., 2004, 6, 2825 CrossRef CAS PubMed; (b) S. Couty, B. Liégault, C. Meyer and J. Cossy, Org. Lett., 2004, 6, 2511 CrossRef CAS PubMed; (c) R. Yanada, S. Obika, T. Inokuma, K. Yanada, M. Yamashita, S. Ohta and Y. Takemoto, J. Org. Chem., 2005, 70, 6972 CrossRef CAS PubMed; (d) D. M. D'Souza, F. Rominger and T. J. J. Müller, Angew. Chem., Int. Ed., 2005, 44, 153 CrossRef PubMed; (e) R. Yanada, S. Obika, Y. Kobayashi, T. Inokuma, M. Oyama, K. Yanada and Y. Takemotoa, Adv. Synth. Catal., 2005, 347, 1632 CrossRef CAS.
- (a) I. Fatima, I. Ahmad, S. A. Nawaz, A. Malik, N. Afza, G. Luttfullah and M. I. Choudhary, Heterocycles, 2006, 68, 1421 CrossRef CAS; (b) M. S. Majik, C. Rodrigues, S. Mascarenhas and L. D'Souza, Bioorg. Chem., 2014, 54, 89 CrossRef CAS PubMed; (c) R. Shimazawa, M. Kuriyama and R. Shirai, Bioorg. Med. Chem. Lett., 2008, 18, 3350 CrossRef CAS PubMed.
- (a) J. M. Tour and E. Negishi, J. Am. Chem. Soc., 1985, 107, 8289 CrossRef CAS; (b) E. Negishi, S. Ma, J. Amanfu, C. Copéret, J. A. Miller and J. M. Tour, J. Am. Chem. Soc., 1996, 118, 5919 CrossRef CAS; (c) K. Itoh, M. Miura and M. Nomura, Tetrahedron Lett., 1992, 33, 5369 CrossRef CAS.
- A byproduct, N-(2-iodophenyl)-3-methoxy-N-(4-nitrobenzyl)acrylamide, was generated using other palladium catalysts. The byproduct was contributed from the addition of methanol to the terminal alkyne.
- (a) S. T. Gadge and B. M. Bhanage, Synlett, 2013, 918 Search PubMed; (b) B. Gabriele, G. Salerno, L. Veltri, M. Costa and C. Massera, Eur. J. Org. Chem., 2001, 4607 CrossRef CAS.
- (a) D. M. Kim and B. S. Yang, Exp. Mol. Med., 2003, 35, 421 CrossRef CAS PubMed; (b) A. Kubo, K. Nakagawa, R. K. Varma, N. K. Conrad, J. Q. Cheng, W. C. Lee, J. R. Testa, B. E. Johnson, F. J. Kaye and M. J. Kelley, Clin. Cancer Res., 1999, 5, 4279 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Compound characterization and spectral (1H and 13C NMR) data. See DOI: 10.1039/c5ob01863c |
| This journal is © The Royal Society of Chemistry 2016 |