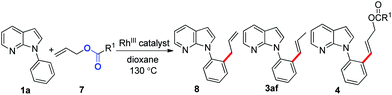
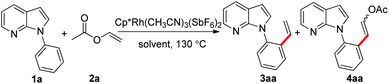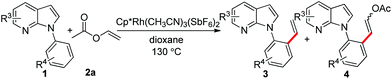Rhodium(III)-catalyzed C–C coupling of 7-azaindoles with vinyl acetates and allyl acetates†
Shuai-Shuai
Li
abc,
Cheng-Qi
Wang
a,
Hui
Lin
a,
Xiao-Mei
Zhang
b and
Lin
Dong
*a
aKey Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry, West China School of Pharmacy, Sichuan University, Chengdu 610041, China
bKey Laboratory for Asymmetric Synthesis and Chiral Technology of Sichuan Province, Chengdu Institute of Organic Chemistry, Chinese Academy of Sciences, Chengdu 610041, China
cGraduate School of the Chinese Academy of Sciences, Beijing 100049, China. E-mail: dongl@scu.edu.cn
First published on 4th November 2015
Abstract
The behaviour of electron-rich alkenes with 7-azaindoles in rhodium(III)-catalyzed C–H activation is investigated. Various substituted vinyl acetates and allyl acetates as coupling partners reacted smoothly providing a wide variety of 7-azaindole derivatives, and the selectivity of the coupling reaction is alkene-dependent. In addition, the approaches of rhodium(III)-catalyzed dehydrogenative Heck-type reaction (DHR) and carbonylation reaction were quite novel and simple.
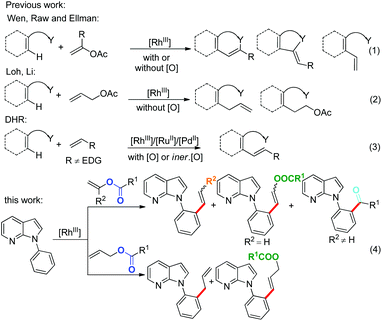
The azaindole ring system, particularly the structural moiety of 7-azaindoles, is one of the most valuable units present in many biologically active natural products.1–4 However, only limited methods have been developed for functional group modifications of 7-azaindoles with the aid of transition metals.5,6 Given the unique structure of 7-azaindoles, the development of new protocols for efficient utilities of the motif could be highly desirable.
Recently, a number of methodologies were established to generate a diversity of heterocyclic scaffolds via rhodium-catalyzed C–C coupling between arenes and alkenes.7–9 However, it was reported that rhodium(III)-catalyzed C–H activation of substituted vinyl acetates mostly afforded functionalized olefinated products, in which vinyl acetate serves as an acetylene equivalent (Scheme 1, eqn (1)),8 while it was also found that allyl acetates generally provided allylated or alkylated products (Scheme 1, eqn (2)).9 To our knowledge, transition-metal-catalyzed dehydrogenative Heck-type reactions (DHR) (Scheme 1, eqn (3)) usually involve the oxidative coupling of sp2 C–H and reactive electron-withdrawing olefins or ethylene by using external oxidants,10 while there are only a few examples of the use of internal oxidants.11 Therefore, there is increased interest in investigating DHR of electron-rich alkenes and 7-azaindoles owing to its usability and accessibility.
Herein, we describe a novel rhodium(III)-catalyzed C–H activation of 7-azaindoles with less-reactive electron-rich alkenyl esters and allyl acetates to provide unexpected DHR and carbonyl products besides other 7-azaindole derivatives, and the selectivity of the coupling reaction is dependent on alkenes (Scheme 1, eqn (4)).
Results and discussion
We began our study by examining the potential reaction of 7-azaindole 1a and vinyl acetate 2a (Table 1). Only trace amounts of the olefinated product 3aa and the unexpected DHR product 4aa were detected when the reaction was carried out in the [Cp*RhCl2]2/AgSbF6 catalyst system, which was reported by Ellman for the preparation of styrene derivatives (entry 1).8c To our delight, the highly efficient preformed cationic Cp*Rh(CH3CN)3(SbF6)2 catalyst could increase the yields of each product (entry 2). Indeed, the solvent was very essential for producing products selectively, and dioxane was the optimal solvent (entries 3–5). Satisfyingly, the yield of 4aa was improved enormously when we increased the loading of 2a as the co-solvent (entry 6). In addition, the reaction gave the best total yields of 98% even when the catalyst loading was reduced to 3 mol% (entry 7). However, no better results were obtained with the attempt to further lower the loading of the catalyst (entries 8 and 9).| Entry | Catalyst/[equiv.] | Additive | Solvent | Yieldb [%] | |
|---|---|---|---|---|---|
| 3aa | 4aa | ||||
| a Reaction conditions unless otherwise specified: 0.1 mmol of 1a, 1.0 mmol of 2a, 1.0 mL of solvent, 130 °C, Ar atmosphere. b Isolated yield. c The same conditions as reported: 5 mol% of [Cp*RhCl2]2, 20 mol% of AgSbF6, 0.5 mL of 2a, 0.5 mL of MeOH, 24 h. d 0.5 mL of 2a. | |||||
| 1c | [Cp*RhCl2]2/0.05 | AgSbF6 | MeOH | 8 | 7 |
| 2 | Cp*Rh(CH3CN)3(SbF6)2/0.05 | — | Toluene | 10 | 32 |
| 3 | Cp*Rh(CH3CN)3(SbF6)2/0.05 | — | Dioxane | 15 | 35 |
| 4 | Cp*Rh(CH3CN)3(SbF6)2/0.05 | — | DMF | 8 | 10 |
| 5 | Cp*Rh(CH3CN)3(SbF6)2/0.05 | — | tAmOH | 22 | 20 |
| 6d | Cp*Rh(CH3CN)3(SbF6)2/0.05 | — | Dioxane | 25 | 70 |
| 7d | Cp*Rh(CH3CN)3(SbF6)2/0.03 | — | Dioxane | 38 | 60 |
| 8d | Cp*Rh(CH3CN)3(SbF6)2/0.02 | — | Dioxane | 22 | 40 |
| 9d | Cp*Rh(CH3CN)3(SbF6)2/0.01 | — | Dioxane | 10 | 25 |
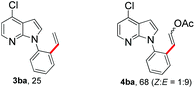
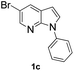
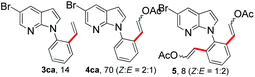
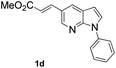
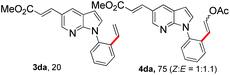
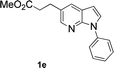
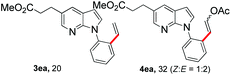
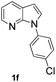
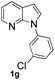
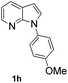
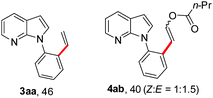
With the optimized conditions in hand, we first examined the scope of substituted 7-azaindoles. As shown in Table 2, a range of 7-azaindoles with diverse N-aryl groups were explored. The substrates with halogens on the pyridine ring (1b and 1c) reacted smoothly with vinyl acetate 2a delivering the corresponding products in excellent total yields, and giving DHR products and olefinated products in better ratios. In addition, the dialkenylation product 5 was obtained in the reaction. Moreover, the functionalized alkene substituted substrate 1d and the alkyl substituted substrate 1e also showed good reactivity. In contrast, azaindole fused with an array of diversely substituted phenyl rings (1f, 1g and 1h) under the optimized conditions to deliver the corresponding products in excellent total yields (entries 5–7).
| Entry | 7-Azaindole 1 | Yieldb [%] |
|---|---|---|
| a General reaction conditions unless otherwise specified: 0.1 mmol of 1, 0.5 mL of 2a, 3 mol% of Cp*Rh(CH3CN)3(SbF6)2, 1.0 mL of dioxane, 130 °C, Ar atmosphere. b Isolated yield. Ratios of Z/E are given within parentheses and were determined by 1H NMR analysis. | ||
| 1 |
|
|
| 2 |
|
|
| 3 |
|
|
| 4 |
|
|
| 5 |
|
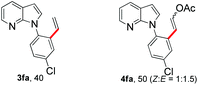
|
| 6 |
|
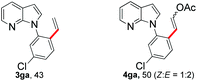
|
| 7 |
|
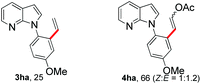
|
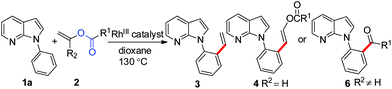
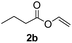
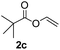
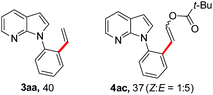
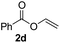
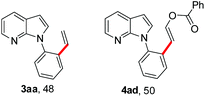
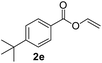
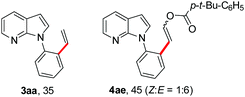
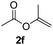
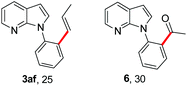
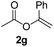
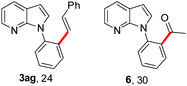
To further highlight the applicability of this procedure, we explored its scope with respect to various substituted electron-rich alkenyl esters (Table 3). The present process showed wide substrate tolerance to alkenyl esters. As other alkenyl esters were less active than vinyl acetate, we raised the catalyst loading up to 5 mol%. Vinyl butyrate 2b and vinyl pivalate 2c both reacted without incident under the reaction conditions to give the same olefinated product 3aa in 46% and 40% yields, respectively. In contrast, the DHR products 4ab and 4ac were also formed in moderate yields. In addition, vinyl aromatic esters 2d and 2e also have similar high reactivity, providing two kinds of products with total yields up to 98%. Importantly, methyl and phenyl substituents on vinyl (2f and 2g) were well tolerated, and the corresponding ortho-olefination products (3af and 3ag) were constructed effectively. Particularly, acetyl substituted 7-azaindole 6 was generated in this reaction system.12,13
| Entry | Alkene 2 | Yieldb [%] |
|---|---|---|
| a General reaction conditions unless otherwise specified: 0.1 mmol of 1a, 10 equiv. of 2, 5 mol% of Cp*Rh(CH3CN)3(SbF6)2, 1.4 mL of dioxane, 130 °C, Ar atmosphere, RhIII = Cp*Rh(CH3CN)3(SbF6)2. b Isolated yield. Ratios of Z/E are given within parentheses and were determined by 1H NMR analysis. | ||
| 1 |
|
|
| 2 |
|
|
| 3 |
|
|
| 4 |
|
|
| 5 |
|
|
| 6 |
|
|
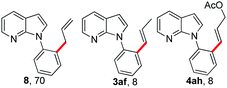
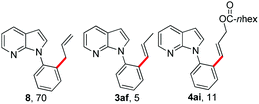
To our delight, allyl electrophiles 7a and 7b were tolerated in this reaction both giving the allylation product 8 (which can be transformed into the more stable olefinated product 3af) in 70% yield (Table 4). It is worth mentioning that DHR products (4ah and 4ai) were also observed in the reactions.
In order to better illustrate the synthetic utility of the DHR products, further transformations were conducted (Scheme 2). Surprisingly, a benzaldehyde derivative 9 was formed when the DHR product 4aa was treated with NaOH in air, while the enol tautomerism product phenylacetaldehyde was not observed (eqn (5)). However, only a trace amount of product 9 and a mixture of unidentified products were observed when the reaction was performed under an Ar atmosphere. In contrast, DHR products 4ah reacted smoothly under the same conditions to give the corresponding hydrolysis product 10 in 99% yield (eqn (6)). This evidence indicates that the enol as the hydrolysis product of 4aa is just an unstable intermediate which could be further oxidized by oxygen to provide benzaldehyde 9.13,14
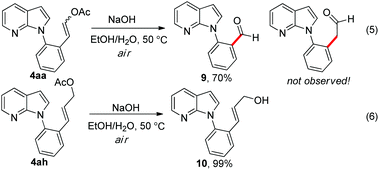 | ||
Scheme 2 Synthetic applications of the DHR products. Reaction conditions: NaOH (2 equiv.), EtOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 50 °C, under air, 3 h. 1), 50 °C, under air, 3 h. | ||
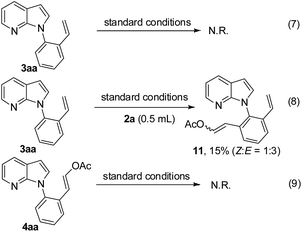
Moreover, to figure out the relationship between the DHR and olefinated products, some experiments were conducted (Scheme 3). When 3aa was explored as a substrate under the standard conditions in the presence or absence of vinyl acetate 2a, 4aa was not observed (eqn (7) and (8)). In contrast, the reaction did not take place at all when 4aa was used as a substrate (eqn (9)). These indicate that 3aa and 4aa have no relationship in this catalytic system.
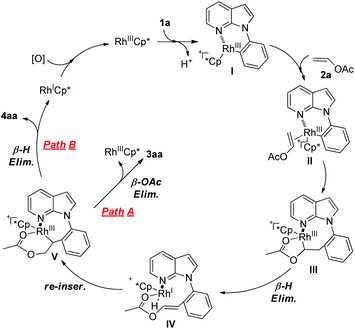
We proposed a plausible mechanism based on these results (Scheme 4).8c,12,13,15 The pathway begins with C–H activation to form a six-membered rhodacycle species I. Then, vinyl acetate coordinates to the rhodacycle species I to form intermediate II. Regioselective insertion of vinyl acetate into the Rh–C bond of intermediate II gives a rhodacycle species III, which undergoes β-H elimination to give rhodium-hydride IV. Reinsertion of the Rh–H bond provides a more stable seven-membered metallacycle V. Elimination of acetate affords the styrene product 3aa (path A). Intermediate V might undergo β-H elimination to afford 4aa and Rh(I) species, and the latter can be reoxidized to the rhodium(III) catalyst to allow the reaction cycle to continue (path B). However, the oxidant is still unclear right now, and further mechanistic studies will be required to elucidate the mechanism for oxidation. In addition, although the mechanistic details of the production of the product 6 are not clear, we have proposed a plausible mechanism in the ESI.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13
13
Conclusion
In summary, we have developed a novel rhodium(III)-catalyzed C–H activation of 7-azaindoles and various electron-donating olefins. Furthermore, diverse substituted alkenyl esters and allyl acetates are well tolerated, giving access to a range of different 7-azaindole derivatives. In this way, we have extended the scope of DHR and carbonyl reaction. We anticipate that this approach will find applications in the selective diversification of heterocyclic frameworks. Further investigation of the catalytic mechanism is underway in our laboratory.Experimental
General remarks
NMR data were obtained for 1H at 400 MHz or 600 MHz, and for 13C at 100 MHz or 151 MHz. Chemical shifts were reported in ppm from tetramethylsilane with the solvent resonance as the internal standard in CDCl3 or DMSO-d6 solutions. ESI HRMS was recorded on a Waters SYNAPT G2 and a Waters XEVO G2 Q-ToF. UV detection was monitored at 220 nm. TLC was performed on glass-backed silica plates. Column chromatography was performed on silica gel (200–300 mesh), eluting with ethyl acetate and petroleum ether.General procedure for the preparation of the 7-azaindole derivatives
1-Phenyl-1H-pyrrolo[2,3-b]pyridine 1a (0.1 mmol, 19.4 mg), vinyl acetate 2a (0.5 mL) and Cp*Rh(CH3CN)3(SbF6)2 (2.5 mg, 3.0 mol%) were stirred in dioxane (1.0 mL) in a sealed tube at 130 °C for 30 h. After completion, the reaction mixture was purified by flash chromatography eluting with ethyl acetate and petroleum ether (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) to give the product 3aa as a colorless oil (8.3 mg, 38%), and with ethyl acetate and petroleum ether (1
50) to give the product 3aa as a colorless oil (8.3 mg, 38%), and with ethyl acetate and petroleum ether (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to give the product 4aa as a colorless oil (16.6 mg, 60%).
10) to give the product 4aa as a colorless oil (16.6 mg, 60%).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 4aa′ = 5
4aa′ = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 4).
30 h, 60% yield; 1H NMR (600 MHz, CDCl3): δ 8.33 (t, J = 3.8 Hz, 2H), 8.03–7.96 (m, 3H), 7.77 (d, J = 12.7 Hz, 1H), 7.66 (d, J = 6.2 Hz, 1H), 7.46 (dd, J = 11.0, 7.5 Hz, 2H), 7.41 (dd, J = 15.7, 6.0 Hz, 4H), 7.28 (t, J = 3.1 Hz, 2H), 7.13 (dt, J = 11.0, 6.9 Hz, 3H), 6.63 (dd, J = 20.7, 3.4 Hz, 2H), 6.01 (d, J = 12.7 Hz, 1H), 5.35 (d, J = 7.4 Hz, 1H), 2.09 (s, 3H), 2.08 (s, 3H) ppm. ESI HRMS: calcd for C17H14N2O2 + H 279.1134, found 279.1124.
4).
30 h, 60% yield; 1H NMR (600 MHz, CDCl3): δ 8.33 (t, J = 3.8 Hz, 2H), 8.03–7.96 (m, 3H), 7.77 (d, J = 12.7 Hz, 1H), 7.66 (d, J = 6.2 Hz, 1H), 7.46 (dd, J = 11.0, 7.5 Hz, 2H), 7.41 (dd, J = 15.7, 6.0 Hz, 4H), 7.28 (t, J = 3.1 Hz, 2H), 7.13 (dt, J = 11.0, 6.9 Hz, 3H), 6.63 (dd, J = 20.7, 3.4 Hz, 2H), 6.01 (d, J = 12.7 Hz, 1H), 5.35 (d, J = 7.4 Hz, 1H), 2.09 (s, 3H), 2.08 (s, 3H) ppm. ESI HRMS: calcd for C17H14N2O2 + H 279.1134, found 279.1124.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 4ba′ = 9
4ba′ = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1).
30 h, 68% yield; 1H NMR (400 MHz, CDCl3): δ 8.21 (d, J = 5.1 Hz, 1H), 8.01 (d, J = 7.7 Hz, 1H), 7.48 (dd, J = 7.8, 4.0 Hz, 1H), 7.44 (s, 2H), 7.33 (d, J = 3.2 Hz, 1H), 7.19–7.13 (m, 2H), 6.73 (d, J = 3.2 Hz, 1H), 5.30 (d, J = 7.4 Hz, 1H), 2.22 (s, 1H), 2.12 (s, 3H) ppm. ESI HRMS: calcd for C17H13ClN2O2 + Na 335.0563, found 335.0558, 337.0560.
1).
30 h, 68% yield; 1H NMR (400 MHz, CDCl3): δ 8.21 (d, J = 5.1 Hz, 1H), 8.01 (d, J = 7.7 Hz, 1H), 7.48 (dd, J = 7.8, 4.0 Hz, 1H), 7.44 (s, 2H), 7.33 (d, J = 3.2 Hz, 1H), 7.19–7.13 (m, 2H), 6.73 (d, J = 3.2 Hz, 1H), 5.30 (d, J = 7.4 Hz, 1H), 2.22 (s, 1H), 2.12 (s, 3H) ppm. ESI HRMS: calcd for C17H13ClN2O2 + Na 335.0563, found 335.0558, 337.0560.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 4ca′ = 1
4ca′ = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2).
24 h, 70% yield; 1H NMR (400 MHz, CDCl3): δ 8.34 (s, 1H), 8.10 (d, J = 6.5 Hz, 1H), 8.00 (d, J = 7.7 Hz, 1H), 7.77 (d, J = 12.8 Hz, 1H), 7.66 (d, J = 7.2 Hz, 1H), 7.51–7.44 (m, 1H), 7.42 (d, J = 4.0 Hz, 3H), 7.38 (dd, J = 13.5, 7.0 Hz, 1H), 7.29 (s, 2H), 7.16 (d, J = 7.4 Hz, 1H), 6.60 (d, J = 3.3 Hz, 1H), 6.56 (d, J = 3.3 Hz, 1H), 5.94 (d, J = 12.7 Hz, 1H), 5.29 (d, J = 7.3 Hz, 1H), 2.12 (s, 3H), 2.09 (s, 2H) ppm. ESI HRMS: calcd for C17H13BrN2O2 + Na 379.0058, found 379.0066, 381.0049.
2).
24 h, 70% yield; 1H NMR (400 MHz, CDCl3): δ 8.34 (s, 1H), 8.10 (d, J = 6.5 Hz, 1H), 8.00 (d, J = 7.7 Hz, 1H), 7.77 (d, J = 12.8 Hz, 1H), 7.66 (d, J = 7.2 Hz, 1H), 7.51–7.44 (m, 1H), 7.42 (d, J = 4.0 Hz, 3H), 7.38 (dd, J = 13.5, 7.0 Hz, 1H), 7.29 (s, 2H), 7.16 (d, J = 7.4 Hz, 1H), 6.60 (d, J = 3.3 Hz, 1H), 6.56 (d, J = 3.3 Hz, 1H), 5.94 (d, J = 12.7 Hz, 1H), 5.29 (d, J = 7.3 Hz, 1H), 2.12 (s, 3H), 2.09 (s, 2H) ppm. ESI HRMS: calcd for C17H13BrN2O2 + Na 379.0058, found 379.0066, 381.0049.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 5′ = 2
5′ = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1).
24 h, 8% yield; 1H NMR (400 MHz, CDCl3): δ 8.33 (s, 2H), 7.99 (t, J = 7.6 Hz, 2H), 7.77 (d, J = 12.8 Hz, 1H), 7.66 (d, J = 5.0 Hz, 1H), 7.49–7.37 (m, 6H), 7.28 (s, 2H), 7.13 (dd, J = 10.7, 6.9 Hz, 3H), 6.65 (d, J = 3.2 Hz, 1H), 6.61 (d, J = 3.2 Hz, 1H), 6.01 (d, J = 12.8 Hz, 1H), 5.35 (d, J = 7.3 Hz, 1H), 2.09 (s, 2H), 2.08 (s, 4H) ppm. ESI HRMS: calcd for C21H17BrN2O4 + Na 463.0269, found 463.0279, 465.0264.
1).
24 h, 8% yield; 1H NMR (400 MHz, CDCl3): δ 8.33 (s, 2H), 7.99 (t, J = 7.6 Hz, 2H), 7.77 (d, J = 12.8 Hz, 1H), 7.66 (d, J = 5.0 Hz, 1H), 7.49–7.37 (m, 6H), 7.28 (s, 2H), 7.13 (dd, J = 10.7, 6.9 Hz, 3H), 6.65 (d, J = 3.2 Hz, 1H), 6.61 (d, J = 3.2 Hz, 1H), 6.01 (d, J = 12.8 Hz, 1H), 5.35 (d, J = 7.3 Hz, 1H), 2.09 (s, 2H), 2.08 (s, 4H) ppm. ESI HRMS: calcd for C21H17BrN2O4 + Na 463.0269, found 463.0279, 465.0264.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 4da′ = 1.1
4da′ = 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1).
12 h, 75% yield; 1H NMR (600 MHz, CDCl3): δ 8.49 (s, 2H), 8.17 (dd, J = 9.4, 1.3 Hz, 2H), 8.01 (d, J = 7.8 Hz, 1H), 7.86 (d, J = 4.0 Hz, 1H), 7.83 (d, J = 4.0 Hz, 1H), 7.78 (d, J = 12.7 Hz, 1H), 7.67 (d, J = 7.4 Hz, 1H), 7.48 (dd, J = 11.2, 4.9 Hz, 1H), 7.43 (dd, J = 16.7, 9.1 Hz, 4H), 7.39 (t, J = 7.6 Hz, 1H), 7.32 (t, J = 3.4 Hz, 2H), 7.16 (d, J = 7.4 Hz, 1H), 6.69 (d, J = 3.5 Hz, 1H), 6.66 (d, J = 3.5 Hz, 1H), 6.51 (d, J = 3.3 Hz, 1H), 6.49 (d, J = 3.3 Hz, 1H), 5.97 (d, J = 12.8 Hz, 1H), 5.32 (d, J = 7.4 Hz, 1H), 3.82 (s, 6H), 2.11 (s, 3H), 2.09 (s, 3H) ppm. ESI HRMS: calcd for C21H18N2O4 + H 363.1345, found 363.1354.
1).
12 h, 75% yield; 1H NMR (600 MHz, CDCl3): δ 8.49 (s, 2H), 8.17 (dd, J = 9.4, 1.3 Hz, 2H), 8.01 (d, J = 7.8 Hz, 1H), 7.86 (d, J = 4.0 Hz, 1H), 7.83 (d, J = 4.0 Hz, 1H), 7.78 (d, J = 12.7 Hz, 1H), 7.67 (d, J = 7.4 Hz, 1H), 7.48 (dd, J = 11.2, 4.9 Hz, 1H), 7.43 (dd, J = 16.7, 9.1 Hz, 4H), 7.39 (t, J = 7.6 Hz, 1H), 7.32 (t, J = 3.4 Hz, 2H), 7.16 (d, J = 7.4 Hz, 1H), 6.69 (d, J = 3.5 Hz, 1H), 6.66 (d, J = 3.5 Hz, 1H), 6.51 (d, J = 3.3 Hz, 1H), 6.49 (d, J = 3.3 Hz, 1H), 5.97 (d, J = 12.8 Hz, 1H), 5.32 (d, J = 7.4 Hz, 1H), 3.82 (s, 6H), 2.11 (s, 3H), 2.09 (s, 3H) ppm. ESI HRMS: calcd for C21H18N2O4 + H 363.1345, found 363.1354.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 4ea′ = 2
4ea′ = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1).
12 h, 32% yield; 1H NMR (400 MHz, CDCl3): δ 8.19 (s, 2H), 8.02–7.98 (m, 1H), 7.85–7.80 (m, 2H), 7.78 (d, J = 12.8 Hz, 1H), 7.67–7.63 (m, 1H), 7.43 (ddd, J = 15.5, 10.4, 6.7 Hz, 6H), 7.26 (d, J = 3.2 Hz, 2H), 7.15 (d, J = 7.4 Hz, 1H), 6.59 (d, J = 3.5 Hz, 1H), 6.55 (d, J = 3.5 Hz, 1H), 6.01 (d, J = 12.8 Hz, 1H), 5.35 (d, J = 7.4 Hz, 1H), 3.68 (s, 6H), 3.07 (td, J = 7.7, 2.5 Hz, 4H), 2.69 (td, J = 7.7, 3.1 Hz, 4H), 2.10 (s, 3H), 2.09 (s, 3H) ppm. ESI HRMS: calcd for C21H20N2O4 + H 365.1501, found 365.1501.
1).
12 h, 32% yield; 1H NMR (400 MHz, CDCl3): δ 8.19 (s, 2H), 8.02–7.98 (m, 1H), 7.85–7.80 (m, 2H), 7.78 (d, J = 12.8 Hz, 1H), 7.67–7.63 (m, 1H), 7.43 (ddd, J = 15.5, 10.4, 6.7 Hz, 6H), 7.26 (d, J = 3.2 Hz, 2H), 7.15 (d, J = 7.4 Hz, 1H), 6.59 (d, J = 3.5 Hz, 1H), 6.55 (d, J = 3.5 Hz, 1H), 6.01 (d, J = 12.8 Hz, 1H), 5.35 (d, J = 7.4 Hz, 1H), 3.68 (s, 6H), 3.07 (td, J = 7.7, 2.5 Hz, 4H), 2.69 (td, J = 7.7, 3.1 Hz, 4H), 2.10 (s, 3H), 2.09 (s, 3H) ppm. ESI HRMS: calcd for C21H20N2O4 + H 365.1501, found 365.1501.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 4fa′ = 1.5
4fa′ = 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1).
24 h, 50% yield; 1H NMR (400 MHz, CDCl3): δ 8.32 (s, 2H), 7.98 (t, J = 7.5 Hz, 3H), 7.78 (d, J = 12.8 Hz, 1H), 7.63 (s, 1H), 7.38 (d, J = 7.4 Hz, 2H), 7.33 (d, J = 8.5 Hz, 2H), 7.25 (s, 2H), 7.18 (d, J = 7.4 Hz, 1H), 7.16–7.09 (m, 2H), 6.65 (d, J = 3.3 Hz, 1H), 6.62 (d, J = 3.3 Hz, 1H), 5.94 (d, J = 12.8 Hz, 1H), 5.28 (d, J = 7.4 Hz, 1H), 2.14 (s, 2H), 2.09 (s, 3H) ppm. ESI HRMS: calcd for C17H13ClN2O2 + Na 335.0563, found 335.0558, 337.0592.
1).
24 h, 50% yield; 1H NMR (400 MHz, CDCl3): δ 8.32 (s, 2H), 7.98 (t, J = 7.5 Hz, 3H), 7.78 (d, J = 12.8 Hz, 1H), 7.63 (s, 1H), 7.38 (d, J = 7.4 Hz, 2H), 7.33 (d, J = 8.5 Hz, 2H), 7.25 (s, 2H), 7.18 (d, J = 7.4 Hz, 1H), 7.16–7.09 (m, 2H), 6.65 (d, J = 3.3 Hz, 1H), 6.62 (d, J = 3.3 Hz, 1H), 5.94 (d, J = 12.8 Hz, 1H), 5.28 (d, J = 7.4 Hz, 1H), 2.14 (s, 2H), 2.09 (s, 3H) ppm. ESI HRMS: calcd for C17H13ClN2O2 + Na 335.0563, found 335.0558, 337.0592.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 4ga′ = 2
4ga′ = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1).
30 h, 50% yield; 1H NMR (400 MHz, CDCl3): δ 8.33 (d, J = 3.0 Hz, 2H), 7.98 (t, J = 7.5 Hz, 2H), 7.93 (d, J = 8.6 Hz, 1H), 7.75 (d, J = 12.8 Hz, 1H), 7.57 (d, J = 8.4 Hz, 1H), 7.47 (s, 1H), 7.40 (dd, J = 13.9, 9.1 Hz, 3H), 7.24 (d, J = 3.9 Hz, 2H), 7.14 (dt, J = 12.2, 4.0 Hz, 3H), 6.65 (d, J = 3.4 Hz, 1H), 6.61 (d, J = 3.4 Hz, 1H), 5.95 (d, J = 12.8 Hz, 1H), 5.30 (d, J = 7.4 Hz, 1H), 2.09 (s, 2H), 2.07 (s, 3H) ppm. ESI HRMS: calcd for C17H13ClN2O2 + Na 335.0563, found 335.0557.
1).
30 h, 50% yield; 1H NMR (400 MHz, CDCl3): δ 8.33 (d, J = 3.0 Hz, 2H), 7.98 (t, J = 7.5 Hz, 2H), 7.93 (d, J = 8.6 Hz, 1H), 7.75 (d, J = 12.8 Hz, 1H), 7.57 (d, J = 8.4 Hz, 1H), 7.47 (s, 1H), 7.40 (dd, J = 13.9, 9.1 Hz, 3H), 7.24 (d, J = 3.9 Hz, 2H), 7.14 (dt, J = 12.2, 4.0 Hz, 3H), 6.65 (d, J = 3.4 Hz, 1H), 6.61 (d, J = 3.4 Hz, 1H), 5.95 (d, J = 12.8 Hz, 1H), 5.30 (d, J = 7.4 Hz, 1H), 2.09 (s, 2H), 2.07 (s, 3H) ppm. ESI HRMS: calcd for C17H13ClN2O2 + Na 335.0563, found 335.0557.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 4ha′ = 1.2
4ha′ = 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1).
24 h, 66% yield; 1H NMR (600 MHz, CDCl3): δ 8.34–8.31 (m, 2H), 8.00–7.96 (m, 2H), 7.75 (d, J = 12.7 Hz, 1H), 7.58 (d, J = 2.9 Hz, 1H), 7.35 (d, J = 8.6 Hz, 1H), 7.29 (d, J = 8.6 Hz, 1H), 7.25–7.24 (m, 2H), 7.13 (dd, J = 7.5, 4.9 Hz, 2H), 7.12–7.09 (m, 2H), 6.97 (d, J = 2.9 Hz, 1H), 6.94 (d, J = 2.9 Hz, 1H), 6.93 (d, J = 2.8 Hz, 1H), 6.62 (d, J = 3.5 Hz, 1H), 6.59 (d, J = 3.5 Hz, 1H), 5.92 (d, J = 12.8 Hz, 1H), 5.25 (d, J = 7.4 Hz, 1H), 3.89 (s, 2H), 3.88 (s, 3H), 2.13 (s, 2H), 2.07 (s, 3H) ppm. ESI HRMS: calcd for C18H16N2O3 + H 309.1239, found 309.1229.
1).
24 h, 66% yield; 1H NMR (600 MHz, CDCl3): δ 8.34–8.31 (m, 2H), 8.00–7.96 (m, 2H), 7.75 (d, J = 12.7 Hz, 1H), 7.58 (d, J = 2.9 Hz, 1H), 7.35 (d, J = 8.6 Hz, 1H), 7.29 (d, J = 8.6 Hz, 1H), 7.25–7.24 (m, 2H), 7.13 (dd, J = 7.5, 4.9 Hz, 2H), 7.12–7.09 (m, 2H), 6.97 (d, J = 2.9 Hz, 1H), 6.94 (d, J = 2.9 Hz, 1H), 6.93 (d, J = 2.8 Hz, 1H), 6.62 (d, J = 3.5 Hz, 1H), 6.59 (d, J = 3.5 Hz, 1H), 5.92 (d, J = 12.8 Hz, 1H), 5.25 (d, J = 7.4 Hz, 1H), 3.89 (s, 2H), 3.88 (s, 3H), 2.13 (s, 2H), 2.07 (s, 3H) ppm. ESI HRMS: calcd for C18H16N2O3 + H 309.1239, found 309.1229.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 11′ = 3
11′ = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1).
48 h, 15% yield; 1H NMR (600 MHz, CDCl3): δ 8.32 (d, J = 3.8 Hz, 1H), 8.02 (d, J = 8.2 Hz, 1H), 8.00–7.96 (m, 1H), 7.73 (d, J = 12.7 Hz, 1H), 7.67–7.61 (m, 1H), 7.57 (d, J = 7.6 Hz, 1H), 7.49 (t, J = 7.7 Hz, 1H), 7.43 (t, J = 7.6 Hz, 1H), 7.16 (s, 1H), 7.15–7.11 (m, 1H), 7.08 (d, J = 7.2 Hz, 1H), 6.68 (d, J = 13.5 Hz, 1H), 6.05–5.94 (m, 1H), 5.76–5.64 (m, 2H), 5.10 (d, J = 11.0 Hz, 1H), 5.01 (d, J = 7.3 Hz, 1H), 2.21 (s, 1H), 2.05 (s, 3H) ppm. ESI HRMS: calcd for C19H16N2O2 + H 305.1290, found 305.1288.
1).
48 h, 15% yield; 1H NMR (600 MHz, CDCl3): δ 8.32 (d, J = 3.8 Hz, 1H), 8.02 (d, J = 8.2 Hz, 1H), 8.00–7.96 (m, 1H), 7.73 (d, J = 12.7 Hz, 1H), 7.67–7.61 (m, 1H), 7.57 (d, J = 7.6 Hz, 1H), 7.49 (t, J = 7.7 Hz, 1H), 7.43 (t, J = 7.6 Hz, 1H), 7.16 (s, 1H), 7.15–7.11 (m, 1H), 7.08 (d, J = 7.2 Hz, 1H), 6.68 (d, J = 13.5 Hz, 1H), 6.05–5.94 (m, 1H), 5.76–5.64 (m, 2H), 5.10 (d, J = 11.0 Hz, 1H), 5.01 (d, J = 7.3 Hz, 1H), 2.21 (s, 1H), 2.05 (s, 3H) ppm. ESI HRMS: calcd for C19H16N2O2 + H 305.1290, found 305.1288.
Acknowledgements
We are grateful for the financial support from the NSFC (21202106, 21582138), Sichuan University “985 project-Science and technology innovation platform for novel drug development”.Notes and references
- (a) J. J. Song, J. T. Reeves, F. Gallou, Z. Tan, N. K. Yee and C. H. Senanayake, Chem. Soc. Rev., 2007, 36, 1120 RSC; (b) D. Song, H. Schmider and S. Wang, Org. Lett., 2002, 4, 4049 CrossRef CAS PubMed; (c) F. Popowycz, S. Routier, B. Joseph and J.-Y. Mérour, Tetrahedron, 2007, 63, 1031 CrossRef CAS; (d) H. L. Milton, M. V. Wheatley, A. M. Slawin and J. D. Woollins, Inorg. Chem. Commun., 2004, 7, 1106 CrossRef CAS; (e) J.-Y. Mérour, S. Routier, F. Suzenet and B. Joseph, Tetrahedron, 2013, 69, 4767 CrossRef; (f) Q. Wu, J. A. Lavigne, Y. Tao, M. D'Iorio and S. Wang, Chem. Mater., 2001, 13, 71 CrossRef CAS; (g) A. Echalier, K. Bettayeb, Y. Ferandin, O. Lozach, M. Clément, A. Valette, F. Liger, B. Marquet, J. C. Morris and J. A. Endicott, J. Med. Chem., 2008, 51, 737 CrossRef CAS PubMed; (h) Q. Wu, A. Hook and S. Wang, Angew. Chem., Int. Ed., 2000, 39, 3933 CrossRef CAS; (i) Y.-S. Tung, M. S. Coumar, Y.-S. Wu, H.-Y. Shiao, J.-Y. Chang, J.-P. Liou, P. Shukla, C.-W. Chang, C.-Y. Chang and C.-C. Kuo, J. Med. Chem., 2011, 54, 3076 CrossRef CAS PubMed; (j) N. M. Barl, E. Sansiaume-Dagousset, K. Karaghiosoff and P. Knochel, Angew. Chem., Int. Ed., 2013, 52, 10093 CrossRef CAS PubMed.
- L. C. Cooper, G. G. Chicchi, K. Dinnell, J. M. Elliott, G. J. Hollingworth, M. M. Kurtz, K. L. Locker, D. Morrison, D. E. Shaw and K.-L. Tsao, Bioorg. Med. Chem. Lett., 2001, 11, 1233 CrossRef CAS PubMed.
- A. Mullard, Nat. Rev. Drug Discovery, 2012, 11, 91 CrossRef CAS PubMed.
- A. Zambon, I. Niculescu-Duvaz, D. Niculescu-Duvaz, R. Marais and C. J. Springer, Bioorg. Med. Chem. Lett., 2012, 22, 789 CrossRef CAS PubMed.
- (a) Y. Chen, S. Guo, K. Li, J. Qu, H. Yuan, Q. Hua and B. Chen, Adv. Synth. Catal., 2013, 355, 711 CrossRef CAS; (b) P. Kannaboina, K. Anilkumar, S. Aravinda, R. A. Vishwakarma and P. Das, Org. Lett., 2013, 15, 5718 CrossRef CAS PubMed.
- (a) M. P. Huestis and K. Fagnou, Org. Lett., 2009, 11, 1357 CrossRef CAS PubMed; (b) G. Qian, X. Hong, B. Liu, H. Mao and B. Xu, Org. Lett., 2014, 16, 5294 CrossRef CAS PubMed; (c) S.-S. Li, C.-Q. Wang, H. Lin, X.-M. Zhang and L. Dong, Org. Lett., 2015, 17, 3018 CrossRef CAS PubMed.
- (a) F. W. Patureau and F. Glorius, J. Am. Chem. Soc., 2010, 132, 9982 CrossRef CAS PubMed; (b) J. Park, N. K. Mishra, S. Sharma, S. Han, Y. Shin, T. Jeong, J. S. Oh, J. H. Kwak, Y. H. Jung and I. S. Kim, J. Org. Chem., 2015, 80, 1818 CrossRef CAS PubMed; (c) H. Wang, N. Schröder and F. Glorius, Angew. Chem., Int. Ed., 2013, 52, 5386 CrossRef CAS PubMed; (d) H. Wang, B. Beiring, D. G. Yu, K. D. Collins and F. Glorius, Angew. Chem., Int. Ed., 2013, 52, 12430 CrossRef CAS PubMed; (e) A. S. Tsai, M. Brasse, R. G. Bergman and J. A. Ellman, Org. Lett., 2011, 13, 540 CrossRef CAS PubMed; (f) F. Wang, G.-Y. Song and X. Li, Org. Lett., 2010, 12, 5430 CrossRef CAS PubMed.
- For selected examples of rhodium-catalyzed oxidative olefination, see: (a) M. Zhang, H.-J. Zhang, T. Han, W. Ruan and T.-B. Wen, J. Org. Chem., 2015, 80, 620 CrossRef CAS PubMed; (b) N. J. Webb, S. P. Marsden and S. A. Raw, Org. Lett., 2014, 16, 4718 CrossRef CAS PubMed; (c) K. D. Otley and J. A. Ellman, Org. Lett., 2015, 17, 1332 CrossRef CAS PubMed; (d) X.-T. Li, X. Gong, M. Zhao, G.-Y. Song, J. Deng and X. Li, Org. Lett., 2011, 13, 5808 CrossRef CAS PubMed; (e) Y. J. Takahama, Y. Shibata and K. Tanaka, Chem. – Eur. J., 2015, 21, 9053 CrossRef CAS PubMed.
- (a) C. Feng, D. Feng and T.-P. Loh, Org. Lett., 2013, 15, 3670 CrossRef CAS PubMed; (b) C. Feng, D. Feng and T.-P. Loh, Chem. Commun., 2015, 51, 342 RSC.
- For selected DHR, see: (a) J. Chen, G. Song, C.-L. Pan and X. Li, Org. Lett., 2010, 12, 5426 CrossRef CAS PubMed; (b) Z. Shi, N. Schröder and F. Glorius, Angew. Chem., Int. Ed., 2012, 51, 8092 CrossRef CAS PubMed; (c) N. Umeda, K. Hirano, T. Satoh and M. Miura, J. Org. Chem., 2009, 74, 7094 CrossRef CAS PubMed; (d) S. H. Park, J. Y. Kim and S. Chang, Org. Lett., 2011, 13, 2372 CrossRef CAS PubMed; (e) C. Feng and T.-P. Loh, Chem. Commun., 2011, 47, 10458 RSC; (f) F. W. Patureau and F. Glorius, J. Am. Chem. Soc., 2010, 132, 9982 CrossRef CAS PubMed; (g) Y. Lu, D.-H. Wang, K. M. Engle and J.-Q. Yu, J. Am. Chem. Soc., 2010, 132, 5916 CrossRef CAS PubMed; (h) G. Cai, Y. Fu, Y. Li, X. Wan and Z. Shi, J. Am. Chem. Soc., 2007, 129, 7666 CrossRef CAS PubMed; (i) J. Wu, X. Cui, L. Chen, G. Jiang and Y. Wu, J. Am. Chem. Soc., 2009, 131, 13888 CrossRef CAS PubMed; (j) J. Pospech and L. Ackermann, Org. Lett., 2011, 13, 4153 CrossRef PubMed.
- DHR with internal oxidants, see: (a) S. Rakshit, C. Grohmann, T. Besset and F. Glorius, J. Am. Chem. Soc., 2011, 133, 2350 CrossRef CAS PubMed; (b) B. Li, J.-F. Ma, N.-C. Wang, H.-L. Feng, S.-S. Xu and B. Wang, Org. Lett., 2012, 14, 736 CrossRef CAS PubMed.
- (a) X.-F. Yang, X.-H. Hu and T.-P. Loh, Org. Lett., 2015, 17, 1481 CrossRef CAS PubMed; (b) F. Pan, Z.-Q. Lei, H. Wang, H. Li, J. Sun and Z.-J. Shi, Angew. Chem., Int. Ed., 2013, 52, 2063 CrossRef CAS PubMed; (c) R. Qiu, L. Zhang, C. Xu, Y. Pan, H. Pang, L. Xu and H. Li, Adv. Synth. Catal., 2015, 357, 1229 CrossRef CAS.
- For more details see the ESI.†.
- B. Hu, Y.-F. Li, Z.-J. Li and X.-B. Meng, Org. Biomol. Chem., 2013, 11, 4138 CAS.
- P. Mamone, G. Danoun and L. J. Gooßen, Angew. Chem., Int. Ed., 2013, 52, 6704 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ob02096d |
| This journal is © The Royal Society of Chemistry 2016 |