Synthesis of some novel steroidal 1,2,4,5-tetraoxanes
Archana M. Das* and
Manash P. Hazarika
Natural Products Chemistry Division, CSIR-North East Institute of Science & Technology, Jorhat, 785 006, Assam, India. E-mail: archanads2@gmail.com
First published on 4th February 2015
Abstract
A facile synthesis of A-ring manipulated C-20 methyl carboxylate steroid derivative with unsymmetrical dispiro 1,2,4,5-tetraoxanes has been focused herein via acid catalyzed cyclocondensation of bis-epidioxy ketone. Novel stable unsymmetrical steroidal based spirocycloalkane 1,2,4,5 tetraoxane 8 has been developed from 3β-acetoxy-pregn-5(6), 16(17)-diene-20-one (16-dehydropregnenolone acetate, i.e. 16-DPA) 1 via metal-mediated halogenation as a key reaction.
1. Introduction
Tetroxanes are cyclic peroxides which have attracted considerable attention in clinical practice as antimalarial and antimicrobial drugs. Many studies reported that tetroxanes have similar antimalarial modes of action compared to the naturally occurring peroxides such as artemisinin and its derivatives.1–3 In the past few decades, chemists and researchers put their attention to develop the organic peroxides in the field of drug design due to certain representatives of these compounds exhibiting antimalarial4–9 and antitumor10–14 activities. This has stimulated the development of several molecules of these types as depicted in various studies. It is pertinent to note that cyclic compounds such as tetraoxanes and trioxanes are considered as the most promising synthetic peroxides having antimalarial and antimicrobial activities. Some of these exhibit high antimalarial activity and antibacterial activity15–18 compared to the natural peroxide artemisinin, a potent antimalarial drug.19 Nowadays, the design of explosives using cyclic peroxides is of particular interest. In addition to these, the synthesis of unsymmetrical tetroxane has become one of the promising areas towards the development of antimalarial drugs.20 Syntheses of peroxides (tetroxanes) are mainly based on the cyclocondensation reaction of ketones/aldehydes with steroids and its intermediates13,20 or alicyclic gem-bis-hydroperoxides,21 and aliphatic/alicyclic gem-bis-hydroperoxides.15,22Malaria has been considered as a serious threat to the health and economic prosperity of the human race in recent years. It is estimated that approximately 300 million clinical cases were observed and more than 2.5 million people die from this disease each year. Due to resistance of the vector (Anopheles mosquito) to insecticides and ongoing spread of the drug-resistant strains of Plasmodium falciparum against chloroquine and other clinically used drugs, there is considerable interest worldwide in new effective anti-malarial drug development.23–25
Symmetric tetraoxanes are limited in number and non-symmetric tetraoxanes could offer more opportunity for the selective incorporation of various functional groups on the tetraoxane scaffold. Several factors, such as the structure of ketones, temperature, solvent, pH and the catalyst concentration of the substrate of the bis-peroxides, affect the synthesis of 1,2,4,5-tetroxanes.
In continuation to our work on steroid transformations,26 we developed a potential method for the metal mediated halogenation of 16-DPA and its relatives using reagents such as MnO2–TMSCl–AcOH. This reaction has been utilized to introduce C-20 methyl carboxylate group in a steroid molecule [Scheme 1].
Our effort has been given to introduce spirocycloalkane 1,2,4,5-tetroxanes that possess significantly higher stability than that of their 1,2,4-trioxane or 1,2,4-trioxolane counterparts.27,28 It is pertinent to note that to date, no reports on the tetroxane in pregnane-like structures are available except some reports on cholestane-like structure.14 In the synthetic route, we have utilized our metal mediated halogenation technique26 to construct the C-20 methyl carboxylate side chain in D-ring and its derivatives, minimize the side effects associated with this class of compounds and make it a soft drug-like structure.
2. Results and discussion
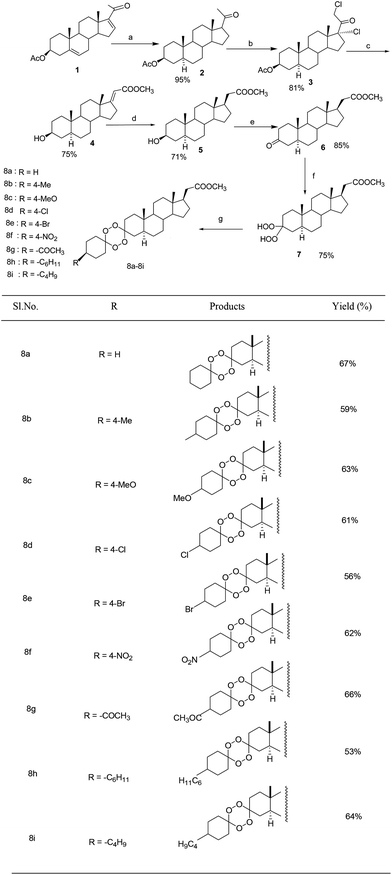
As depicted in Scheme 1, 16-DPA was hydrogenated in presence of Pd/C to furnish the product 2, which was subjected to metal mediated halogenation reaction using MnO2–TMSCl–AcOH system to furnish 17α,21-dichloro-20-oxopregnane 3 in high yield. The product was characterized by direct comparison with the authentic materials.26,28,29 This compound 3 was reacted with alkaline methanolic solution to give the 3β-hydroxy Favorskii rearrangement product 4.30,31 Catalytic hydrogenation of 4 in the presence of Pd/C provided the hydrogenated compound 5 in high yield. PCC oxidation of 5 in methylene chloride gave the corresponding 3-oxosteroid 6. Conversion of the compound 3-oxoandrostan 6 to bis-hydroperoxy androstan 7 was carried out by using 30% H2O2 in acetonitrile at 0 °C,14 and the reaction 7 was carried out with cyclohexanone/substituted cyclohexanone in the presence of conc. H2SO4 in CH3CN to afford target compounds 8a–8i.3. Conclusion
A facile and novel route towards the synthesis of 1,2,4,5-tetroxane 8a–8i from 16-dehydropregenolone acetate i.e., 16-DPA 1 using acid catalyzed cyclocondensation of bis-epidioxy tetraoxanes also with a C-20 methyl carboxylate side chain in ring D was developed. The method affords the target compounds with good yield (53–67%). Here also the acid acts both as catalyst and co-solvent, which influences both the formation of tetraoxanes and the stability of the peroxides during the experiment.4. Experimental
4.1. General remarks
All the chemicals used were of the reagent grade of E. Merck and were used without further purification. The progress of each reaction was monitored using Merck thin layer chromatography silica gel 60 F254. Melting points were measured with a Buchi B-540 melting point apparatus and are uncorrected. IR spectra were recorded with a Perkin-Elmer model 2000 series FT-IR spectrometer for solutions in chloroform. Infrared absorbance is reported in reciprocal centimeters (cm−1). 1H and 13C NMR spectra were recorded using a Bruker DPX (300 MHz) spectrometer using CDCl3 or DMSO-d6 as solvent with tetramethylsilane (TMS) as internal standard on ppm scale (d). Multiplicity of the resonance peaks are indicated as singlet (s), broad singlet (bs), doublet (d), triplet (t), quartet (q) and multiplet (m). Mass spectrometric analysis was performed by positive mode electro spray ionization with Bruker Esquire 3000 LC-MS instrument. Elemental analysis was carried out using Varian CHN analyzer (Perkin-Elmer 2400 II).4.2. Experimental methodology and chemistry
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ethyl acetate and hexane as eluent. The product obtained was pregnenolone acetate 2.
10 ethyl acetate and hexane as eluent. The product obtained was pregnenolone acetate 2.Yield: 950 mg (95%); melting point (mp.) 172 °C. The observed 1H and 13C NMR data (300 MHz, CDCl3) agree well with the previously reported values.26 IR (CHCl3): 1735, 1700, 1450, 1200 cm−1; MS (ESI) m/z: 360 (M)+. Anal. calcd for C23H36O3: C, 77.66; H, 10.00. Found: C, 77.49; H, 9.65.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 ethyl acetate and hexane as eluent.
40 ethyl acetate and hexane as eluent.Yield: 405 mg (81%); mp. 160 °C. The observed 1H and 13C NMR data (300 MHz, CDCl3) agree well with the previously reported values.26 IR (CHCl3): 1730, 1710, 1445, 1200 cm−1; MS (ESI) m/z: 428 (M)+. Anal. calcd for C23H34O3Cl2: C, 64.49; H, 7.94. Found: C, 64.46; H, 7.8; αD = +35° (CHCl3, 22 °C and 0.1%).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) at room temperature for a period of 6 hours. The reaction was monitored using TLC. Then the reaction mixture was poured into cold water (300 mL), acidified with 30% citric acid solution and extracted with chloroform (5 × 100 mL). The organic layer was dried over anhydrous sodium sulfate and evaporated under reduced pressure to get the solid crude product. The product was purified by column chromatography to get the desired pure product 4 over silica gel using 1
15) at room temperature for a period of 6 hours. The reaction was monitored using TLC. Then the reaction mixture was poured into cold water (300 mL), acidified with 30% citric acid solution and extracted with chloroform (5 × 100 mL). The organic layer was dried over anhydrous sodium sulfate and evaporated under reduced pressure to get the solid crude product. The product was purified by column chromatography to get the desired pure product 4 over silica gel using 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ethyl acetate and hexane as eluent.
2 ethyl acetate and hexane as eluent.Yield: 375 mg (75%); mp. 165 °C; IR (cm−1): 3400, 1730, and 1250. The observed 1H and 13C NMR data (300 MHz, CDCl3) agree well with the previously reported values.26 MS (ESI) m/z: 346 [M]+. Anal. calcd for C22H34O3: C, 76.30; H, 9.83; found: C, 76.21; H, 9.76.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ethyl acetate and hexane as eluent.
3 ethyl acetate and hexane as eluent.Yield: 284 mg (71%); mp. 172 °C; 1H NMR (CDCl3): 0.8 (s, 3H, Me), 1.2 (s, 3H, Me), 0.9–2.1 (m, 23H, –CH and –CH2), 2.2 (m, 1H, 3-OH), 3.4 (s, 3H, OMe), 3.5 (m, 1H, H-3), 2.3 (s, 1H, H-20); 13C NMR: δ 15.2, 16.4, 21.4, 27.7, 29.7, 31.5, 32.0, 32.2, 36.6, 36.9, 37.8, 40.6, 46.3, 49.9, 56.8, 65.0, 73.8, 170.5; IR (cm−1): 3400 (b), 1735, 1450, 1250; MS (ESI) m/z: 348 [M]+. Anal. calcd for C22H36O3: C, 75.86; H, 10.34; found: C, 75.60; H, 10.18.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ethyl acetate
5 ethyl acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane. After completion of the reaction (2 hours), the reaction mixture was diluted with 5 volumes of anhydrous ether and allowed to pass through neutral alumina. The ether was distilled under reduced pressure to get the crude product. The product was purified by column chromatography to get the desired pure product 6 over silica gel using 1
hexane. After completion of the reaction (2 hours), the reaction mixture was diluted with 5 volumes of anhydrous ether and allowed to pass through neutral alumina. The ether was distilled under reduced pressure to get the crude product. The product was purified by column chromatography to get the desired pure product 6 over silica gel using 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ethyl acetate and hexane as eluent.
5 ethyl acetate and hexane as eluent.Yield: 170 mg (85%); mp. 267 °C; IR (cm−1): 1735, 1715, 1450, 1250; 1H NMR (CDCl3): δ 0.8 (s, 3H, Me), 1.0 (s, 3H, Me), 0.9–2.1 (m, 23H and –CH2), 3.4 (s, 3H, Me), 2.3 (s, 1H, H-20); 13C NMR: δ 13.4, 16.4, 18.8, 19.3, 20.6, 21.4, 27.7, 30.7, 31.5, 31.9, 32.1, 36.7, 36.9, 37.8, 38.0, 40.6, 46.3, 49.9, 56.9, 64.9, 170.6, 205.2; MS (ESI) m/z: 346 [M]+. Anal. calcd for C22H34O3: C, 76.3; H, 9.82; found: C, 76.01; H, 9.79.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ethyl acetate and hexane as eluent.
2 ethyl acetate and hexane as eluent.Yield: 75 mg (75%); mp. 287 °C; IR (cm−1): 1727.3, 1446.3, 1263, 754.6; 1H NMR (CDCl3): δ 0.8 (s, 3H, Me-19), 1.0 (s, 3H, Me-18), 1.1–2.1 (m, 23H and –CH2), 3.5 (s, 3H, Me), 3.7 (s, methyl ester), 9.6 (broad singlet, –OOH); 13C NMR: δ 12.5, 16.0, 24.4, 28.1, 12.5, 16.0, 24.4, 28.1, 28.8, 31.7, 32.1, 32.2, 35.8, 38.0, 42.1, 46.6, 51.4, 56.6, 110, 174.6; MS (ESI) m/z: 396 [M]+. Anal. calcd for C22H36O6: C, 66.66; H, 9.09; found: C, 66.45; H, 9.03.
Yield: 201 mg (67%); mp. 185 °C; IR (cm−1): 2932, 1711, 1448.3, 1198, 773; 1H NMR (CDCl3): δ 0.8 (s, 3H, Me-19), 1.2 (s, 3H, Me-18), 0.9–2.0 (m, 23H and –CH2), 3.2 (s, 3H, Me), 3.6 (s, methyl ester); 13C NMR: δ 22.1, 22.3, 22.5, 22.7, 23.3, 24.0, 25.1, 26.0, 26.6, 27.1, 27.5, 31.7, 33.7, 37.7, 41.9, 42.1, 58.2, 58.6, 85.0, 97.7, 109.1, 174.0; MS (ESI) m/z: 476 [M]+. Anal. calcd for C28H44O6: C, 70.59; H, 9.30; found: C, 70.32; H, 9.11.
Acknowledgements
The authors thank the Director of CSIR-North East Institute of Science & Technology, Jorhat, Assam for providing facilities and valuable advice and also gratefully acknowledge the financial assistance supported by DST, New Delhi, for awarding Fast Track Young Scientist Award, EEOES and CSIR, New Delhi, India.References
- A. K. Bhattacharjee, K. A. Carvalho, D. Opsenica and B. A. Solaja, J. Serb. Chem. Soc., 2005, 70, 329–345 CrossRef CAS.
- S. Tonmunphean, A. Wijitkosoom and Y. Tantirungrotechai, Bioorg. Med. Chem., 2004, 12, 2005–2012 CrossRef CAS PubMed.
- R. Amewu, A. V. Stachulski, S. A. Ward, N. G. Berry, P. G. Bray, J. Davies, G. Labat, L. Vivas and P. M. O'Neill, Org. Biomol. Chem., 2006, 4, 4431–4436 CAS.
- P. M. O'Neil and G. H. Posner, J. Med. Chem., 2004, 47, 2945–2964 CrossRef PubMed.
- M. H. Gelb, Curr. Opin. Chem. Biol., 2007, 11, 440–445 CrossRef CAS PubMed.
- H.-X. Jin, H.-H. Liu, Q. Zhang and Y. Wu, Tetrahedron Lett., 2005, 46, 5767–5769 CrossRef CAS PubMed.
- H.-X. Jin, Q. Zhang, H.-S. Kim, Y. Wataya, H.-H. Liu and Y. Wu, Tetrahedron, 2006, 62, 7699–7711 CrossRef CAS PubMed.
- F. Najjar, L. Gorrichon, M. Baltas, C. André-Barrés and H. Vial, Org. Biomol. Chem., 2005, 3, 1612–1614 CAS.
- G. L. Ellis, R. Amewu, C. Hall, K. Rimmer, S. A. Ward and P. M. O'Neill, Bioorg. Med. Chem. Lett., 2008, 18, 1720–1724 CrossRef CAS PubMed.
- V. M. Dembitsky, T. A. Gloriozova and V. V. Poroikov, Med. Chem., 2007, 7, 571–589 CAS.
- V. M. Dembitsky, Eur. J. Med. Chem., 2008, 43, 223–251 CrossRef CAS PubMed.
- N. Terzic, D. Opsenica, D. Milic, B. Tinant, K. S. Smith, W. K. Milhous and B. A. Solaja, J. Med. Chem., 2007, 50, 5118–5127 CrossRef CAS PubMed.
- D. Opsenica, D. E. Kyle, W. K. Milhous and B. A. Solaja, J. Serb. Chem. Soc., 2003, 68, 291–302 CrossRef CAS.
- R. Amewu, A. V. Stachulski, S. A. Ward, N. G. Berry, P. G. Bray, J. Davies, G. Labat, L. Vivas and P. M. O'Neill, Org. Biomol. Chem., 2006, 4, 4431–4436 CAS.
- Y. Dong, Y. Tang, J. Chollet, H. Matile, S. Wittlin, S. A. Charman, W. N. Charman, J. S. Tomas, C. Scheurer, C. Snyder, B. Scorneaux, S. Bajpai, S. A. Alexander, X. Wang, M. Padmanilayam, S. R. Cheruku, R. Brun and J. L. Vennerstrom, Bioorg. Med. Chem., 2006, 14, 6368–6382 CrossRef CAS PubMed.
- C. Singh, H. Malik and S. K. Puri, Bioorg. Med. Chem. Lett., 2004, 14, 459–462 CrossRef CAS PubMed.
- J. L. Vennerstrom, S. Arbe-Barnes, R. Brun, S. A. Charman, F. C. K. Chiu and J. Chollet, Nature, 2004, 430, 900–904 CrossRef CAS PubMed.
- P. M. O'Neill, V. E. Barton and S. A. Ward, Molecules, 2010, 15, 1705–1721 CrossRef PubMed.
- N. Kumar, M. Sharma and D. S. Rawat, Curr. Med. Chem., 2011, 18(25), 3889–3928 CrossRef CAS.
- B. A. Solaja, N. Terzic, G. Pocsfalvi, L. Genena, B. Tinant, D. Opsenica and W. K. Milhous, J. Med. Chem., 2002, 45, 3331–3336 CrossRef CAS PubMed.
- D. Opsenica, G. Pocsfalvi, Z. Juranic, B. Tinant, J.-P. Declercq, D. E. Kyle, W. K. Milhous and B. A. Solaja, J. Med. Chem., 2000, 43, 3274–3282 CrossRef CAS PubMed.
- J. Iskra, D. Bonnet-Delpon and J.-P. Begue, Tetrahedron Lett., 2003, 44, 6309–6312 CrossRef CAS.
- K. Zmitek, S. Stavber, M. Zupan, D. Bonnet-Delpon and J. Iskra, Tetrahedron, 2006, 62, 1479–1484 CrossRef CAS PubMed.
- I. Opsenica, D. Opsenica, K. S. Smith, W. K. Milhous and B. A. Solaja, J. Med. Chem., 2008, 51, 2261–2266 CrossRef CAS PubMed.
- A. O. Terentev, A. V. Kutkin, Z. A. Starikova, M. Y. Antipin, Y. N. Ogibin and G. I. Nikishin, Synthesis, 2004, 65, 2356–2366 Search PubMed.
- P. Chowdhury, J. M. Borah, P. Goswami and A. M. Das, Steroids, 2011, 76, 497–501 CrossRef CAS PubMed.
- P. M. O'Neill, R. K. Amewu, G. L. Nixon, E. L. Garah, M. Mungthin, J. Chadwick, A. E. Shone, L. Vivas, H. Lander, V. Barton, S. Muangnoicharoen, P. G. Bray, J. Davies, B. K. Park, S. Wittlin, R. Brun, M. Preschel, K. Zhang and S. A. Ward, Angew. Chem., Int. Ed., 2010, 49, 5693–5697 CrossRef PubMed.
- G. L. Ellis, R. Amewu, S. Sabbani, P. A. Stocks, A. E. Shone, D. Stanford, P. Gibbons, J. Davies, L. Vivas, S. Charnaud, E. Bongard, C. Hall, K. Rimmer, S. L. Maria Jesus, D. Gargallo, S. A. Ward and P. M. O'Neill, J. Med. Chem., 2008, 51, 2170–2177 CrossRef CAS PubMed.
- P. Borah, M. Ahmed and P. K. Chowdhury, J. Chem. Res., 1998, 236–237 RSC.
- P. Borah, M. Ahmed and P. K. Chowdhury, J. Chem. Res., 1998, 1173–1180 Search PubMed.
- P. Goswami, S. Hazarika, A. M. Das and P. K. Chowdhury, Indian J. Chem., 2004, 43, 1275–1281 Search PubMed.
| This journal is © The Royal Society of Chemistry 2015 |