Phenylsilane as a safe, versatile alternative to hydrogen for the synthesis of actinide hydrides†
Abstract
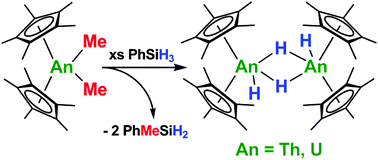
The thorium and uranium dihydride dimer complexes [(C5Me5)2An(H)(μ-H)]2 (An = Th, U) have been easily prepared using phenylsilane, which is an efficient and safer alternative to hydrogen gas. The synthetic utility of this new hydriding method has been demonstrated by the preparation of a variety of organometallic complexes, including, for the first time, (C5Me5)2U(SMe)2, (C5Me5)2Th(C4Ph4), (C5Me5)2U(C4Ph4), (C5Me5)2ThS5, and (C5Me5)2U(bipy) using [(C5Me5)2An(H)(μ-H)]2 (An = Th, U) as multi-electron reductants.

 Please wait while we load your content...
Please wait while we load your content...