Facile microwave-assisted synthesis and effective photocatalytic hydrogen generation of Zn2GeO4 with different morphology†
Min Yang,
Ying Ji,
Wei Liu,
Ying Wang and
Xiaoyang Liu*
State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130012, PR China. E-mail: liuxy@jlu.edu.cn; Fax: +86-431-85168316; Tel: +86-431-85168316
First published on 13th March 2014
Abstract
Single-crystalline hexagonal prism Zn2GeO4 nanorods and hierarchical Zn2GeO4 microspheres have been successfully synthesized via a facile microwave-assisted solution-phase approach. The as-prepared samples were characterized by XRD, SEM, TEM, HRTEM and UV-vis diffuse reflectance spectroscopy. The hierarchical Zn2GeO4 microspheres were found to be constructed of randomly aggregated nanorods which have dimensions of about 50 nm in length and 20 nm in width. Such rhombohedral phase Zn2GeO4 nanorods were found to be 10–20 nm in diameter and ∼200 nm in length. Some influencing factors such as the reaction time, temperature, and the urea were revealed to play crucial roles in the formation of Zn2GeO4 photocatalysts. A possible growth mechanism was proposed based on the experimental results. The rhombohedral phase Zn2GeO4 nanorods exhibited superior photocatalytic activities for the photocatalytic decomposition of water–methanol solution to hydrogen under UV irradiation.
Introduction
Solar-driven photocatalytic hydrogen evolution over semiconductor materials has alluring potential for obtaining clean fuel from renewable resources.1,2 Zinc germanate (Zn2GeO4) has attracted wide attention due to its increasing application in photocatalysis and environmental remediation.3,4 In particular, both experimental results and theoretical analysis indicate that its electronic structure is very suitable for use as a photocatalyst.5 Over the years, various types of Zn2GeO4 nanostructures including nanowires,6 nanorods,7 nanoribbons, and microspheres have been prepared by different methods and showed good activities for various kinds of photocatalytic reactions.8–11 The universality of the photocatalytic function of Zn2GeO4 attracts extensive interest and attention.12 The design and development of highly active photocatalysts has been a challenging research subject in the field.Several solution routes and gas phase evaporation techniques have been developed for the preparation of nanorods, nanoribbons and nanowires of Zn2GeO4.12–14 However, most of the reported routes were related to a complex reaction process.15,16 The surfactant, high-temperature and high-pressure were usually used to control the morphology of the final product, which are not beneficial for obtaining the high-purity and low defect-density product.17 To the best of our knowledge, only several reports have appeared on the fabrication of the 1-D Zn2GeO4. Huang et al. reported the synthesis of Zn2GeO4 nanorods by a surfactant-assisted hydrothermal method.18 Yan and co-workers have prepared ternary Zn2GeO4 nanowires and their branched structures by a chemical vapor transport method.19 These reported procedures usually require long reaction times. A facile and fast solution-based procedure is highly desired for the preparation of Zn2GeO4 photocatalyst materials. In recent years, microwave heating has been widely applied in chemical reactions and materials synthesis.20,21 Zhu et al., reported MW irradiation can promote the nucleation and growth stage of nanocrystals.22 The introduction of microwave to chemical synthesis is important in material science and engineering, and it has been proved to be a fast technology with high yields and reproducibility.23–25 In comparison to conventional heating, the unique microwave dielectric heating mechanism can accelerate the reaction rate much more effectively.20,26–28 However, there were few reports about microwave-assisted synthesis of Zn2GeO4 photo-catalyst materials, especially micro-nano structured exhibited superior photocatalytic activities for the photocatalytic decomposition of water–methanol solution to hydrogen.29
Herein, we report the successful synthesis of Zn2GeO4 nanorods and microspheres through a facile and rapid microwave-assisted solution-phase route. It is shown that the obtained samples indeed exhibit outstanding photocatalytic activities without any co-catalyst for hydrogen production from methanol–water solution. The formation process of the bundle and the correlations between morphology and photocatalytic activities were discussed.
Experimental
Preparation of hexagonal Zn2GeO4 nanorods
The Zn2GeO4 nanorods were synthesized via a facile microwave-assisted solution-phase method without any surfactant assistance. In a typical synthetic procedure, GeO2 and Zn(CH3COO)2·H2O were added to 40 mL of deionized water. The mixture was stirred for 30 min. Then NaOH solution (5 mol L−1) was introduced dropwise to the vigorously stirred solution to adjust pH to 8. After additional agitation for 20 min, the obtained white colloidal precipitate was transferred into a microwave glass vessel, sealed and maintained at 140 °C for 1–10 min using a single-mode Microwave synthesizer, then cooled naturally to room temperature. The products were dried at 80 °C for 12 h. Bulk Zn2GeO4 was prepared by a conventional solid-state reaction. 0.2092 g GeO2 and 0.3256 g ZnO powders were ground and mixed thoroughly in an agate mortar. The resulting mixture was sintered at 1200 °C (ramp rate 5 °C min−1) for 16 h in air to produce bulk Zn2GeO4.12,30Preparation of hierarchical Zn2GeO4 microspheres
1.190 g Zn(NO3)2·6H2O and 3.604 g urea were firstly dissolved in 35 mL deionized water with stirring. Then, 0.209 g GeO2 was added to the solution and the mixture was further stirred for 20 min. The as-obtained white colloidal precipitate was transferred into a microwave glass vessel, sealed and maintained at 170 °C for 1–10 min using a single-mode Microwave synthesizer, Then cooled naturally to room temperature. After the hydrothermal reaction, the products were collected, centrifuged, washed with deionized water for five times. Finally, the white powders were obtained by drying the products at 80 °C.Characterization
The resultant phases of the samples were characterized by X-ray diffraction (XRD, Cu Kα radiation, Rigaku D/max2550VB, Japan). The morphology and structures of samples were observed using scanning electron microscopy (SEM, JSM-6700F, JEOL, Japan) equipped with an energy dispersive X-ray (EDX) spectrometer. The transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) images were obtained on a FEI Tecnai G2S-Twin with a field emission gun operating at 200 kV. The UV/Vis diffuse reflectance spectra were recorded on a Perkin-Elmer Lambda 20 UV/Vis spectrometer. The microwave system was a single-mode Microwave Synthesizer (Biotage AB, Uppsala, Sweden).Photocatalytic performance
The photocatalytic hydrogen evolution reaction was performed in a closed gas-recirculation system equipped with a quartz reaction vessel with inner irradiation. 0.075 g of photocatalyst was dispersed in 75 mL of H2O by magnetic stirring. Prior to the reaction, the system was evacuated by a mechanical pump and then filled with 101 kPa high-purity N2 (>99.99%). This process was repeated three times in order to completely remove O2 from the system. After that, 5 mL of CH3OH was introduced into the reactor with a syringe. The suspension was irradiated with a 125 W ultraviolet mercury lamp (GGZ-125). The temperature of the solution was controlled at room temperature by circulating water. The evolved hydrogen gas was circulated with a microdiaphragm gas pump in the system and its amount was determined by an online gas chromatograph.Results and discussion
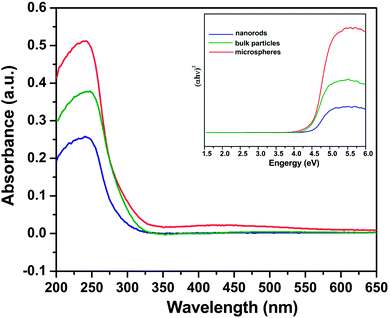
Hydrothermal synthesis has been extensively used to promote preferred growth nanorod, nanoflower and microspheres. However it is hard to control the size and morphology uniformity of the product due to its inhomogeneous environment during long reaction times (∼10–24 hours). Single-mode microwave irradiation can offer a homogenous reaction environment, which has been suggested as an effective tool to obtain photocatalyst with uniform dispersion and size and morphology control. The microwave-assisted hydrothermal reaction time is only 10 min. Fig. 1a shows the XRD patterns of the bulk Zn2GeO4 (1200 °C, 16 h) and Zn2GeO4 samples prepared by the microwave-assisted hydrothermal method. All the diffraction peaks of the Zn2GeO4 sample can be assigned to a pure rhombohedral Zn2GeO4 phase (JCPDS no. 11-0687). No characteristic peaks of other impurities such as ZnO and GeO2 were observed. From the Fig. 1a, it can been seen that the XRD peaks of the Zn2GeO4 nanorods are broader than that of the microspheres and bulk Zn2GeO4 which indicates that the crystalline size of Zn2GeO4 nanorods is smaller than that of microspheres and bulk Zn2GeO4. This result is further confirmed by scanning electron microscopy analysis (Fig. 2). The crystal structure of Zn2GeO4 is shown in Fig. 1b. Zn2GeO4 is a binary compound oxide consisting of ZnO and GeO2 with the space group R3 and has a phenacite structure with lattice constants of a = b = 14.231 Å and c = 9.53 Å. The coordination number of Zn, Ge, O atoms is 4, 4, 3, respectively.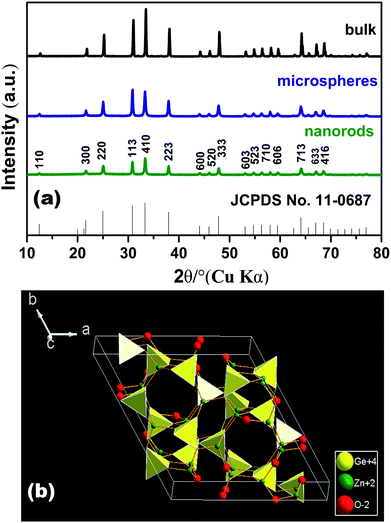 | ||
| Fig. 1 (a) XRD pattern of Zn2GeO4. The standard data of Zn2GeO4 (JCPDS no. 11-0687) as reference; (b) crystal structure of Zn2GeO4 with a unit cell. | ||
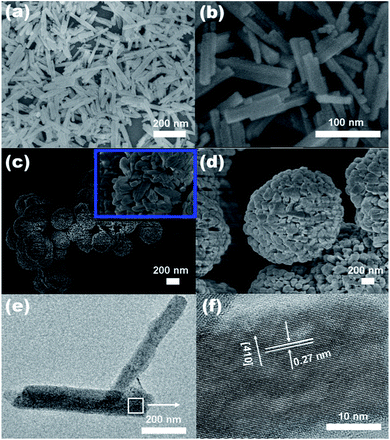
The morphology and microstructure of the as-prepared products were investigated by SEM, FESEM and TEM. Fig. 2a and b, we can clearly see that the as obtained Zn2GeO4 consists of nanorods with smooth surface and perfect prism structure. The length of these nanorods is approximately 100–200 nm, and the diameter ranges from 10 to 20 nm. The sample prepared at 170 °C for 10 min is composed of well-dispersed Zn2GeO4 microspheres with diameters ranging from 1–5 μm (Fig. 2c and d). The inset image in Fig. 2c further shows that the single Zn2GeO4 microsphere is constructed of randomly packed nanorods which are about 20–50 nm in length and 10–20 nm in width. Fig. 2e show TEM image of the rhombohedral Zn2GeO4 nanorods, and the corresponding HRTEM results. The HRTEM image (Fig. 2f) shows well-resolved lattice fringes with an interplanar distance of 0.27 nm corresponding to the (410) d spacing of the rhombohedral Zn2GeO4 structure.31
Influencing factors
In order to reveal factors influencing the formation of nanorods of Zn2GeO4, some controlled experiments have been carefully performed by changing one reaction parameter (reaction temperature, the urea, and reaction time) while the other reaction conditions are kept constant.The effect of reaction temperature
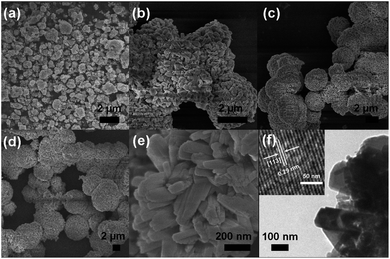
It should be noted that the samples prepared at this temperature were not aligned well and bound tightly. When the temperature was controlled at 100 °C, the product was comprised of nanoparticles agglomeration were represented (Fig. 3a). However, the corresponding XRD pattern shown in Fig. S1a† indicated that the product was a mixed phase of rhombohedral phase Zn2GeO4 (JCPDS file Card no. 11-0687), hexagonal Zn(OH)2 (JCPDS file Card no. 72-2032) and hexagonal GeO2 (JCPDS file Card no. 830548).10 It is found that the variation of reaction temperature greatly changes the product morphology and phase. When the reaction was carried out at 140 °C for 10 min, keeping other experimental parameters constant, hexagonal prism Zn2GeO4 nanorods were produced (Fig. 3c and S1c†). To further obtain the detailed crystal structure of the prismatic Zn2GeO4 nanorods and nanofibers, HRTEM observations were obtained. Fig. 3d show typical TEM images of the hexagonal Zn2GeO4 nanorods, and the corresponding HRTEM results. It is clearly observed that the lattice fringes are parallel to the growth direction of the rod. In Fig. 3d, the lattice fringe of the (300) plane with an interplanar spacing of 0.41 nm is observed parallel to the nanorod direction. According to the above structure information, we can conclude that the hexagonal Zn2GeO4 nanorods grew along the direction of the c-axis of the rhombohedral phenacite-type structure.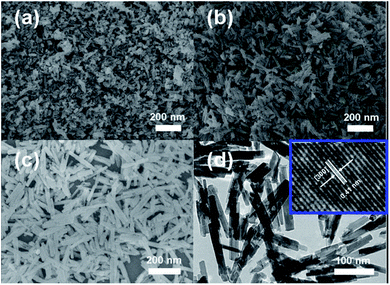 | ||
| Fig. 3 SEM images of the products at different reaction temperatures: (a) 100 °C, (b) 120 °C and 140 °C. TEM images and HRTEM image of the as-prepared Zn2GeO4 nanorod. | ||
The morphology and microstructure of the as-prepared microspheres were investigated by SEM, FESEM and TEM. Fig. 4a shows that the Zn2GeO4 is composed of irregular particles at 100 °C for 10 min. The samples obtained by the microwave-assisted process at 120 and 140 °C for 10 min consist of some agglomerated Zn2GeO4 nanorods (Fig. 4b and c).
The sample prepared at 170 °C for 10 min is composed of well-dispersed Zn2GeO4 microspheres with diameters ranging from 1–5 μm (Fig. 4d). The FESEM image in Fig. 4e further shows that the single Zn2GeO4 microsphere is constructed of randomly packed nanorods which are about 20–50 nm in length and 10–20 nm in width. This result is in agreement with that of XRD analyses (Fig. S2†). Some of microspheres can be found in Fig. 4f, which were further were investigated by TEM. Fig. 4f shows a typical high-resolution transmission electron microscopy image of Zn2GeO4 nanorods. Well-resolved lattice fringes are clearly visible, with an interplanar d spacing of 0.29 nm corresponding to the (113) lattice planes of the rhombohedral Zn2GeO4 structure, indicating good crystallization of the prepared Zn2GeO4 microspheres.
The effect of reaction time and the formation mechanism
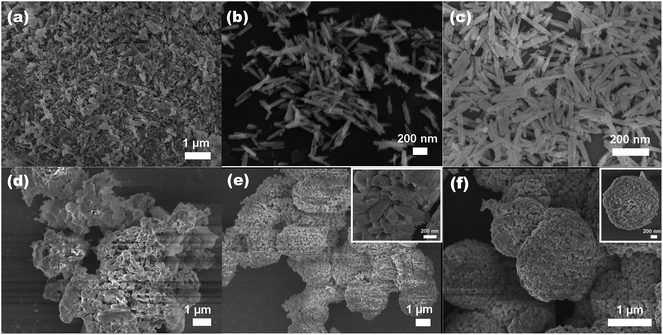
To understand the formation of the Zn2GeO4 nanorods, we carried out time-dependent shape evolution experiments during which samples were collected after different periods of microwave-assisted treatment. At an early stage 1 min, the supersaturated solution leads to the nucleation of Zn2GeO4 and tiny Zn2GeO4 particles are formed from the solution (Fig. 5a). The corresponding XRD pattern shown in Fig. 6a, indicated that the sample was a mixed phase with rhombohedral Zn2GeO4, hexagonal Zn(OH)2 and GeO2. The sample collected 5 min later (Fig. 5b and 6b) shows approximately nanorods with length of 100–200 nm. As the reaction proceeded (Fig. 5c and 6c), the smooth nanorods with perfect prism structure developed.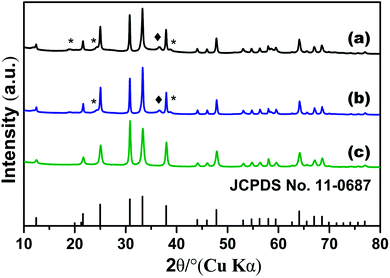 | ||
| Fig. 6 XRD pattern of Zn2GeO4 nanorods 140 °C with different reaction time: (a) 1 min, (b) 5 min and (c) 10 min, *: Zn(OH)2; ♦: GeO2. | ||
On the basis of the experimental results, the formation mechanism for the Zn2GeO4 nanorods is speculated to be as follows: (1) in the synthetic process, GeO2 is hydrolyzed to form GeO32−; (2) when the concentrations of Zn2+ and GeO32− reach the supersaturation degree of Zn2GeO4, small Zn2GeO4 nuclei form according to the following equation: 2Zn2+ +GeO32− + 2OH− = Zn2GeO4 + H2O. (3) These nuclei further grow along the c-axis to produce Zn2GeO4 nanorods.
In order to monitor the morphological evolution and reveal the possible growth mechanism of the Zn2GeO4 microspheres, a series of time-dependent experiments were carefully carried out to gain an insight into the formation process. When the reaction time was controlled at 1 min, the SEM image indicated that the product was comprised of nanoparticles and nanosheets (Fig. 5d). Prolonging the reaction time to 5 min, the yield of Zn2GeO4 microspheres gradually increased which was comprised of nanosheet-assembled hollow spheres (Fig. 5e). Further prolonging to 10 min, well-aligned Zn2GeO4 microspheres were harvested (Fig. 5f). It can be concluded that with increasing reaction time, Zn(OH)2 and GeO2 gradually disappear and more and more Zn2GeO4 microspheres are formed. The corresponding XRD pattern shown in Fig. S3,† From the conditional experiments such as variation of reaction time and temperature, one can find that the products prepared at low reaction temperature or with a short reaction time usually contain the impurities phase of hexagonal Zn(OH)2 and GeO2. With increasing reaction temperature and time, these impurities gradually disappear.
From the time-dependent experiments, it can be concluded that the possible reactions involved in the reaction process could be summarized by eqn (1)–(4).
| CO(NH2)2 + H2O → CO2 + 2NH3 | (1) |
| NH3 + H2O ↔ NH3·H2O ↔ NH4+ + OH− | (2) |
| GeO2 + OH− ↔ HGeO3− | (3) |
| 2Zn2+ + HGeO3− + 3OH− ↔ Zn2GeO4 + 2H2O | (4) |
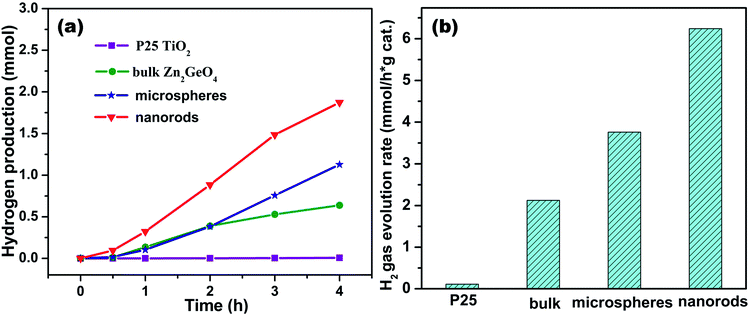
The whole evolution process is illustrated in the scheme of Fig. 7. The photocatalytic performance of the as-prepared Zn2GeO4 nanorods was evaluated by a water splitting reaction in the presence of methanol under UV-light irradiation. For comparison, the photocatalytic activities of the P25 TiO2 and the Zn2GeO4 microspheres were also determined. The hydrogen evolution amounts as a function of reaction time are shown in Fig. 8a. For all samples, the hydrogen production over each catalyst increases proportionally with the reaction time, but all the Zn2GeO4 samples show higher activity than the P25 TiO2.
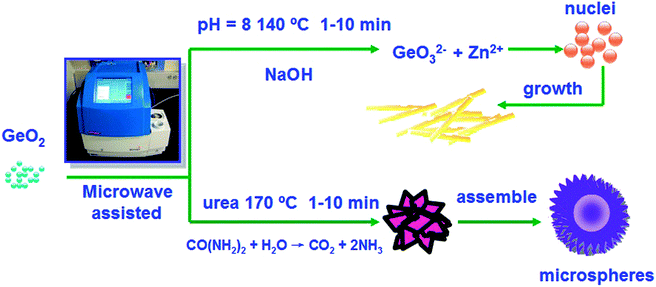 | ||
| Fig. 7 The scheme for synthesizing Zn2GeO4 hexagonal prism Zn2GeO4 nanorods and hierarchical microspheres. | ||
The nanorods and microspheres of photocatalysts Zn2GeO4 were synthesized by a microwave-assisted solution method and investigated for photocatalytic hydrogen generation from water–methanol solution. The controlled blank reaction in the absence of any catalyst showed there was no H2 formation. The photocatalytic activities were also compared with those of the P25 TiO2 and bulk Zn2GeO4 particles. As shown in Fig. 8b, it is found that the nanostructure Zn2GeO4 efficiently improves the photocatalytic activity which exhibits 6.24 mmol g−1 h−1 hydrogen evolution, which is more than 3 times higher than bulks of Zn2GeO4 with calcination. The nanostructured samples are markedly superior in activity to the bulk sample prepared by general solid-state reaction routes. The photocatalytic activity on Zn2GeO4 (microspheres) is also shown for comparison. It is very clear that the photocatalytic activity of microspheres synthesized via microwave-assisted solution is much lower than that of nanorods. For comparison, the photocatalytic performances of Zn2GeO4 and a commercially available TiO2 (P25) were also tested, which showed a superior photocatalytic H2 evolution activity to the benchmark P25 TiO2. Wang and his co-workers synthesized hexagonal nanorods and nanofibers via hydrothermal method, and the photocatalytic activities were also compared with those of the P25 TiO2 and bulk Zn2GeO4 particles.1
The Zn2GeO4 nanorods show the highest activity, and the hydrogen evolution rate is decreased in the order nanorods > microspheres > bulks. Which is nearly 2 times higher than that on the microspheres. The results show that the rod structure of hexagonal Zn2GeO4 nanorods with dominant (110) facets greatly improves photocatalytic hydrogen-evolving properties of the 1-D nanostructures.
The DFT calculation of Zn2GeO4 showed that the top of the valence band is composed of the O 2p orbital, while the bottom of the conduction band is composed of the Ge 4p orbital with a small contribution of the Zn 4s4p orbitals. Such a conduction band is highly dispersive, and therefore leads to large mobility of photoexcited electrons.
In Fig. 8a, the Zn2GeO4 nanorods shows a stable H2 evolution rate of 6.24 mmol g−1 h−1 under UV light. The discussion about different morphologies of samples prepared at different synthetic routes of Zn2GeO4 was listed in Table S1.† Wang et al. reported a highly regular, crystal orientation ordered and tight-binding Zn2GeO4 bundle, which was assembled from hexagonal nanoprism monomers via a triethanolamine (TEA)-induced self-assembly process under mild solvothermal conditions. The H2-producing rate on the Zn2GeO4 bundles is surprisingly up to (4.9 mmol g−1 h−1).1 Liang and his co-workers developed a simple hydrothermal synthesis technique without any organic additives, and unexpectedly obtain hexagonal Zn2GeO4 nanorods and nanofibers with a high aspect ratio. The hexagonal Zn2GeO4 nanorods showed the highest rate of H2 evolution,12 and the evolution rate of hydrogen gas was 6.0 mmol g−1 h−1. Hierarchical Zn2GeO4 hollow spheres were fabricated via a synergistic self-assembly route by Xu and his co-workers.11 The H2-producing rate on the Zn2GeO4 hollow spheres was surprisingly up to (6.23 mmol g−1 h−1). We reported the synthesis of photocatalytic materials with different morphology via microwave-assisted method. The microwave-assisted synthetic material was used as an efficient photocatalytic material for H2 evolution reaction. This work is expected to open up new synthetic avenues towards the preparation of other advanced inorganic nanomaterials with unique structures/composition for various applications, such as photocatalysis and gas sensing.
The optical absorption properties of several Zn2GeO4 samples were investigated. The diffuse absorption spectra in Fig. 9 show that the bulk particles, nanorods, and microspheres, have a bandgap of ca. 4.2, 4.25 and 4.45 eV, respectively, corresponding to an optical absorption edge of about 295, 291, and 278 nm. The light absorption edges of nanostructured Zn2GeO4 samples exhibit a certain extent of blue shift in comparison with those of bulk Zn2GeO4 particles, which can be explained by size effects.32 However, there is no significant difference in the absorption edges of the nanostructured Zn2GeO4 samples. So the difference in the bandgap is not the main reason leading to the discrepancy in their photocatalytic activity. In addition, the optical absorption intensities are decreased in the order microspheres > bulk > nanorods, while the photocatalytic activities are decreased in the order nanorods > microspheres > bulk (Fig. 8b). Apparently, the difference in the photo-absorption is also not the main factor leading to the difference in the photocatalytic activity.
Conclusions
In summary, we present a simple one-pot template-free microwave-assisted route for the synthesis of Zn2GeO4 nanorods and microspheres. The investigation on the evolution formation reveals that the microwave irradiation time played an important role in the formation of the Zn2GeO4 microspheres. High reaction temperature and long reaction time facilitate the formation of pure phase Zn2GeO4 product. For the photocatalytic activities of Zn2GeO4 samples, the Zn2GeO4 nanorods show the highest activity, and the hydrogen evolution rate is decreased in the order nanorods > microspheres > bulks. Moreover, The photocatalytic investigations show that well-formed 1-D Zn2GeO4 nanorods exhibit excellent photocatalytic activities in the photocatalytic decomposition of a water–methanol solution to hydrogen under UV irradiation. Compared to existing solution-phase synthetic methods using hydrothermal reactions, this paper provides a facile, rapid, low-cost pathway to novel Zn2GeO4 nanoarchitectures.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 21271082).Notes and references
- J. Liang, J. Xu, Q. Gu, Y. Zhou, C. Huang, H. Lin and X. Wang, J. Mater. Chem. A, 2013, 1, 7798 CAS.
- A. J. Bard and M. A. Fox, Acc. Chem. Res., 1995, 28, 141 CrossRef CAS.
- S. Yan, J. Wang and Z. Zou, Dalton Trans., 2013, 42, 12975 RSC.
- S. Yan, J. Wang, H. Gao, N. Wang, H. Yu, Z. Li, Y. Zhou and Z. Zou, Adv. Funct. Mater., 2013, 23, 1839 CrossRef CAS.
- J. Wang, C. Yan, S. Magdassi and P. S. Lee, ACS Appl. Mater. Interfaces, 2013, 5, 6793 CAS.
- L. Z. Pei, S. Wang, Y. X. Jiang, Y. K. Xie, Y. Li and Y. H. Guo, CrystEngComm, 2013, 15, 7815 RSC.
- M.-Y. Tsai, S.-H. Huang and T.-P. Perng, J. Lumin., 2013, 136, 322 CrossRef CAS.
- L. Liu, X. Zhao, H. Sun, C. Jia and W. Fan, ACS Appl. Mater. Interfaces, 2013, 5, 6893 CAS.
- J. Liu, G. Zhang, J. C. Yu and Y. Guo, Dalton Trans., 2013, 42, 5092 RSC.
- J. Liu and G. Zhang, CrystEngComm, 2013, 15, 382 RSC.
- J. Liang, J. Xu, J. Long, Z. Zhang and X. Wang, J. Mater. Chem. A, 2013, 1, 10622 CAS.
- J. Liang, Y. Cao, H. Lin, Z. Zhang, C. Huang and X. Wang, Inorg. Chem., 2013, 52, 6916 CrossRef CAS PubMed.
- N. Zhang, S. Ouyang, T. Kako and J. Ye, Chem. Commun., 2012, 48, 9894 RSC.
- S. Takeshita, J. Honda, T. Isobe, T. Sawayama and S. Niikura, J. Solid State Chem., 2012, 189, 112 CrossRef CAS.
- N. Zhang, S. Ouyang, P. Li, Y. Zhang, G. Xi, T. Kako and J. Ye, Chem. Commun., 2011, 47, 2041 RSC.
- J. K. Feng, M. O. Lai and L. Lu, Electrochem. Commun., 2011, 13, 287 CrossRef CAS.
- C. Yan, N. Singh and P. S. Lee, Appl. Phys. Lett., 2010, 96, 053108 CrossRef.
- J. Huang, K. Ding, Y. Hou, X. Wang and X. Fu, ChemSusChem, 2008, 1, 1011 CrossRef CAS PubMed.
- C. Y. Yan and P. S. Lee, J. Phys. Chem. C, 2009, 113, 14135 CAS.
- G. Tan, L. Zhang, H. Ren, S. Wei, J. Huang and A. Xia, ACS Appl. Mater. Interfaces, 2013, 5, 5186 CAS.
- L. C. Mostafa Baghbanzadeh, P. Davide Cozzoli and C. O. Kappe, Angew. Chem., Int. Ed., 2011, 50, 11312 CrossRef PubMed.
- X. X. Zhu, Q. H. Zhang, Y. G. Li and H. Z. Wang, J. Mater. Chem., 2010, 20, 1766 RSC.
- G. A. Tompsett, W. C. Conner and K. S. Yngvesson, ChemPhysChem, 2006, 7, 296 CrossRef CAS PubMed.
- I. Bilecka, I. Djerdj and M. Niederberger, Chem. Commun., 2008, 886 RSC.
- S. H. Kim, S. Y. Lee, G. R. Yi, D. J. Pine and S. M. Yang, J. Am. Chem. Soc., 2006, 128, 10897 CrossRef CAS PubMed.
- T. a. Mulinari, F. a. La Porta, J. Andrés, M. Cilense, J. a. Varela and E. Longo, CrystEngComm, 2013, 15, 7443 RSC.
- X. Li, P. Sun, T. Yang, J. Zhao, Z. Wang, W. Wang, Y. Liu, G. Lu and Y. Du, CrystEngComm, 2013, 15, 2949 RSC.
- M. Sun, D. Li, W. Zhang, Z. Chen, H. Huang, W. Li, Y. He and X. Fu, J. Solid State Chem., 2012, 190, 135 CrossRef CAS.
- F. Zou, X. Hu, Y. Sun, W. Luo, F. Xia, L. Qie, Y. Jiang and Y. Huang, Chemistry, 2013, 19, 6027 CrossRef CAS PubMed.
- Y. Z. Q. Liu, J. H. Kou, X. Y. Chen, Z. P. Tian, J. Gao, S. C. Yan and Z. G. Zou, J. Am. Chem. Soc., 2010, 132, 14385 CrossRef PubMed.
- M. Shang, G. Li, D. Yang, X. Kang, C. Peng, Z. Cheng and J. Lin, Dalton Trans., 2011, 40, 9379 RSC.
- Y. Zheng, F. Duan, J. Wu, L. Liu, M. Q. Chen and Y. Xie, J. Mol. Catal. A: Chem., 2009, 303, 9 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra01041h |
| This journal is © The Royal Society of Chemistry 2014 |