Nanoconfined NaAlH4: prolific effects from increased surface area and pore volume†‡
Thomas K.
Nielsen
a,
Payam
Javadian
a,
Marek
Polanski
b,
Flemming
Besenbacher
c,
Jerzy
Bystrzycki
b,
Jørgen
Skibsted
d and
Torben R.
Jensen
*a
aCenter for Energy Materials, Center for Materials Crystallography, Interdisciplinary Nanoscience Center (iNANO), Department of Chemistry, Aarhus University, Langelandsgade 140, DK-8000 Aarhus, Denmark. E-mail: trj@chem.au.dk
bFaculty of Advanced Technologies and Chemistry, Military University of Technology, 2 Kaliskiego Str., 00-908 Warsaw, Poland
cInterdisciplinary Nanoscience Center (iNANO), Department of Physics and Astronomy, Aarhus University, DK-8000 Aarhus C, Denmark
dInstrument Centre for Solid-State NMR Spectroscopy, Department of Chemistry, Interdisciplinary Nanoscience Center (iNANO), Aarhus University, DK-8000 Aarhus C, Denmark
First published on 22nd October 2013
Abstract
Nanoconfinement is a promising technique to improve the properties of nanomaterials such as the kinetics for hydrogen release and uptake and the stability during cycling. Here we present a systematic study of nanoconfined NaAlH4 in nanoporous scaffolds with increasing surface area and pore volume and almost constant pore sizes in the range of 8 to 11 nm. A resorcinol formaldehyde carbon aerogel was CO2-activated under different conditions and provided aerogels with BET surface areas of 704, 1267 and 2246 m2 g−1 and total pore volumes of 0.91, 1.30 and 2.21 mL g−1, respectively. Nanoconfinement of NaAlH4 was achieved by melt infiltration and 27Al MAS NMR reveals that the respective scaffolds incorporate 68, 82 and 91 wt% NaAlH4, for the above-mentioned samples, while the remaining fraction decomposes to metallic Al indicating that increasing CO2-activation tends to facilitate the infiltration process. The frequencies for the 23Na and 27Al MAS NMR centerband resonances from NaAlH4 vary systematically for the infiltrated samples and are shifted towards higher frequency and become more narrow with increasing degree of CO2 activation of the scaffolds. This new effect is attributed to increasing interactions with conduction electrons from increasingly graphite-/graphene-like scaffolds. The bulk versus nanoconfined ratio of NaAlH4 was investigated using Rietveld refinement, revealing that the majority of added NaAlH4 is confined inside the nanopores. The hydrogen desorption kinetics decreased with increasing surface area and the hydrogen storage capacity is more stable and decreases less during continuous hydrogen release and uptake cycles. In fact, the available amount of hydrogen (2.7 wt% H2) was more than doubled compared to the nanoconfinement in the non-activated carbon aerogel (1.3 wt% H2). Furthermore, it was demonstrated that Ti-functionalization of the CO2-activated aerogels combines the high storage capacity with fast hydrogen release kinetics from NaAlH4 which fully decomposes into Na3AlH6 at T ≤ 100 °C.
1. Introduction
Hydrogen is recognized as a promising future carrier of renewable energy and may play an important role in an environmentally friendly carbon-free energy system.1 Utilization of hydrogen as a substitute for gasoline has the potential to completely eliminate the harmful waste particles and gases from cars, buses and truck exhaust in cities. Hydrogen offers a clean and safe alternative to fossil fuels with water as the only exhaust, which may also reduce the climatic changes we are experiencing in the environment from the exponentially increasing amounts of carbon dioxide in the atmosphere.Solid state hydrogen storage materials such as MgH2, LiBH4 and NaAlH4 offer high gravimetric (ρm = 7.6, 18.5 and 7.5 wt% H2, respectively) and volumetric (ρv = 110, 121 and 94 g H2 L−1) hydrogen storage capacities, which are required for a compact and efficient hydrogen storage system.2,3 However, until now solid state hydrogen storage has suffered from difficulties in combining high storage capacities in light element hydrides with hydrogen release and uptake conditions close to room temperature which is technologically desirable. Therefore, substantial research efforts have been spent on discovering new methods to facilitate hydrogen exchange in these materials under near ambient conditions.4–6 One approach is confinement of metal hydrides in inert mesoporous scaffolds with pore sizes in the range of 2–50 nm, referred to as nanoconfinement.7–10 The metal hydride is usually embedded in the nanoscaffold by melt infiltration or solvent mediated infiltration. The final crystallite size of the hydride may depend on the pore size diameter of the scaffold.11 Previous studies have illustrated that nanoconfinement of MgH2, LiBH4 and NaAlH4 in carbon based scaffolds facilitates improved hydrogen release and uptake kinetics and reversible hydrogen storage capacity and more favorable thermodynamic properties.12–22
Bulk NaAlH4 starts to decompose to Na3AlH6(s), Al(s) and H2(g) upon melting at Tmp ∼ 183 °C and Na3AlH6 further decomposes to NaH(s), Al(s) and H2(g) at T > 240 °C.23 Sodium alanate, NaAlH4, spontaneously infiltrates a nanoporous scaffold using an elevated hydrogen pressure to minimize decomposition reactions.15–17 However, the melt infiltration process is often ineffective and only half of the hydride is nanoconfined while the rest decomposes to Al and presumably NaH.15 In addition, the weight penalty of the scaffold also reduces the gravimetric hydrogen content of the nanocomposite material. This has motivated the present study of CO2 activated resorcinol formaldehyde carbon aerogels with variable surface area and pore volume utilized as nanoscaffolds. Furthermore, a recently proposed catalytic effect from the aerogel surface is also explored.11,24,25
2. Experimental details
2.1. Sample preparation
The resorcinol formaldehyde carbon aerogel (RF-CA) was prepared by mixing 41.618 g resorcinol (Aldrich, 99%), 56.9 mL formaldehyde in water (37 wt% stabilized by ∼10 to 15% methanol, Merck), 56.6 mL of deionised water and 0.65 g Na2CO3 (Aldrich, 99.99%) in a beaker with continuous stirring. The pH value of the final solution was 6.24. Afterwards, the preparation was performed according to previously published procedures.13,15,26 Selected fractions of the prepared carbon aerogel (CA) were CO2-activated in order to increase the surface area.27,28 Monoliths of CA were placed in an Al2O3 crucible, transferred to a tubular oven and heated to 950 °C (heating rate, ΔT/Δt = 6.67 °C min−1) in a CO2 flow. The temperature was kept constant during selected time intervals of 3, 5 and 5.1 hours, and afterwards, the gas flow and the furnace were turned off and the samples were allowed to cool naturally. This approach provided activated CA with variable textures, denoted as CA-3, CA-5 and CA-5.1, respectively. Activated CA-5 and CA-5.1 were treated under similar conditions but were from different synthesis batches. Longer CO2-activation time provides larger surface areas and pore volumes due to aerogel burn off, see Table 1.27–29 Prior to use, all CAs were activated at 400 °C in vacuum for several hours in order to remove moisture and gases from the porous structure. All subsequent handling was performed in a purified argon atmosphere in a glove box.| Sample | CO2 – time (h) | S BET (m2 g−1) | V meso (mL g−1) | V tot (mL g−1) | V micro/Vtot | D max (nm) | NaAlH4a (wt%) | NaAlH4a (vol%) |
|---|---|---|---|---|---|---|---|---|
| a Amounts of NaAlH4 used for the infiltration per weight and volume of the scaffold. b The TiCl3 content was 8.7 wt%. | ||||||||
| CA | 0 | 704 ± 12 | 0.74 ± 0.02 | 0.91 ± 0.02 | 0.19 | 10 ± 0.5 | 34.1 ± 0.02 | 62 ± 2 |
| CA-3 | 3 | 1267 ± 270 | 0.96 ± 0.16 | 1.30 ± 0.27 | 0.28 | 11 ± 0.5 | 41.5 ± 0.02 | 63 ± 13 |
| CA-5 | 5 | 2246 ± 270 | 1.53 ± 0.16 | 2.21 ± 0.27 | 0.29 | 10 ± 0.5 | 51.3 ± 0.02 | 53 ± 7 |
| CA-5.1 | 5.1 | 2236 ± 270 | 1.45 ± 0.16 | 2.11 ± 0.27 | 0.29 | 8 ± 0.5 | 56.5 ± 0.02 | 69 ± 9 |
| CA–Tib | 5.1 | 2041 ± 246 | 1.30 ± 0.14 | 1.93 ± 0.25 | 0.27 | 8 ± 0.5 | 48.6 ± 0.02 | 55 ± 7 |
Aerogel CA-5.1 was functionalized with TiCl3 nanoparticles according to previously published procedures.15 Initially, aerogel monoliths (0.6819 g) were submerged into a solution of 0.1961 g (22.3 wt%) TiCl3 (Aldrich, 99.995%) in 185 mL dry acetone (Aldrich, ≥99.9%). The solution was transferred from the glove box to a Schlenk line system without air exposure and acetone was removed by evaporating under dynamic vacuum. The sample was further activated at 190 °C in dynamic vacuum for 48 hours and this procedure incorporated 8.7 wt% TiCl3 nanoparticles in the CO2 activated CA-5.1, which is denoted as TiCl3 functionalized carbon aerogel, CA–Ti.
Sodium alanate was melt infiltrated by grinding monoliths of CA with NaAlH4 (Aldrich, 90%), obtaining a homogeneous powder. Melt infiltration was performed under inert conditions in a custom-made manually operated steel hydrogenation apparatus by heating to 189 °C (ΔT/Δt = 2.6 °C min−1) reaching hydrogen pressures in the range of 210 to 230 bar. The sample temperature was kept fixed at 189 °C for 15 minutes and then the sample was cooled naturally. The amount of NaAlH4 was calculated to achieve similar pore filling of ∼60 vol% for all nanoconfined samples. A sample of bulk NaAlH4 without CA was melted and cooled under the same conditions as described above and was used as a reference, which is denoted as Na-m.
2.2. Characterization
Characterization of the nanoporous aerogel scaffold material was performed by gas adsorption and desorption using a Nova 2200e surface area and pore size analyzer from Quantachrome. The aerogels were degassed under vacuum for several hours at 300 to 320 °C, prior to the measurements. A full absorption and desorption isotherm was measured in the pressure range of 0 to 1 p/p0 at liquid nitrogen temperatures with nitrogen gas as the adsorbent. Data were analyzed using the t-plot method,30 the Brunauer–Emmett–Teller (BET) method,31 and the Barrett–Joyner–Halenda (BJH) method, and the total volume was calculated from a single point at p/p0 ∼ 1.32Solid-state 23Na and 27Al MAS NMR spectra were recorded on a Varian INOVA-400 (9.39 T) spectrometer, using a homebuilt X-[1H] double-resonance MAS NMR probe for 5 mm o.d. rotors. The NMR experiments were performed at ambient temperature and employed air-tight end-capped zirconia rotors packed with the samples in an argon-filled glove box. 27Al MAS NMR spectra were recorded for the freshly prepared samples whereas the 23Na MAS NMR spectra were acquired for the same samples after one year of storage under argon at −40 °C. The spectra were obtained with spinning speeds of νR = 8–10.0 kHz, short 23Na and 27Al excitation pulses (0.5 μs) for an rf field strength of γB1/2π ≈ 50 kHz, and a 2 s relaxation delay. For the 27Al MAS NMR spectra, the transmitter offset was set to 800 ppm to obtain a similar excitation of the resonances from NaAlH4 and metallic Al. The 23Na and 27Al isotropic chemical shifts are in ppm relative to 1.0 M aqueous solutions of NaCl and AlCl3·6H2O, respectively.
Powder X-ray diffraction was performed with a Rigaku Smartlab diffractometer using Cu wavelength (λ1 = 1.540530 and λ2 = 1.544310, ratio = 0.5). Samples were loaded in glass capillaries (after one year of storage under argon at −40 °C), sealed with glue and transferred to the diffractometer without air exposure. The data were analyzed using Rietveld refinements. The background X-ray scattering was described by linear interpolation between selected points, while Thompson–Cox–Hastings axial divergence symmetry profile functions were used to fit the diffraction peaks and the instrumental resolution function was determined from a PXD pattern of a LaB6 standard. The observed diffraction peaks were modeled using two structural models of NaAlH4 with identical structural parameters (kept fixed) and slightly different unit cell parameters (refined). The average crystallite size for bulk NaAlH4 was kept fixed in accordance with the instrumental resolution while that for nanoconfined NaAlH4 was refined using the Rietveld approach implemented in the Fullprof suite.33
Temperature-Programmed Desorption Mass Spectroscopy, TPD-MS, was performed in a Setaram Sensys Evo differential scanning calorimeter (horizontal position) coupled with a Hiden Analytical quadrupole mass spectrometer (MS) under a constant flow (28 mL min−1) of ultra-high purity helium (<10 ppb O2 and H2O, Air Products) for temperature-programmed desorption mass spectroscopy (TPD-MS) measurements. A powdered sample (<20 mg) was placed in an Al2O3 crucible with a lid and then encapsulated in a protective aluminum crucible. The samples were purged with helium for at least 2 hours and heated in the temperature range of 20 to 450 °C (ΔT/Δt = 1 °C min−1). During the experiment the MS signals at m/z = 2, 18, and 32 were recorded in order to detect H2, H2O and O2. No significant amounts of H2O or O2 were detected.
The cyclic stability during four hydrogen release and uptake cycles for selected samples was studied by Sieverts' measurements. The samples were transferred to a stainless steel autoclave, sealed under argon and attached to the PCTpro 2000 apparatus. Hydrogen desorption data were collected in the temperature range from RT to 200 °C, at p(H2) = 10−2 bar, and with the temperature kept fixed at 100 °C for 4 h, at 150–152 °C for 4 h and at 200–203 °C for 8 h (ΔT/Δt = 0.5 °C min−1). Hydrogen absorption was performed at p(H2) = 89 to 92 bar and a temperature of 160 °C (ΔT/Δt = 5 °C min−1) during 10 hours and then the sample was cooled naturally to RT. All handling of NaAlH4 containing samples, activated CA and all sample preparations were performed in argon filled gloveboxes to avoid air exposure.
3. Results and discussion
3.1. CO2 activated resorcinol formaldehyde carbon aerogels
Carbon aerogel (CA) scaffolds can be synthesized with specific pore size distributions and a CA with a maximum value of the pore size distribution, Dmax = 10 nm, was selected for this study.11 The morphology of CA can be systematically designed by activation in CO2 and scaffolds with gradually increasing specific surface areas, total pore volumes and fraction of micropores (Vmicro/Vtot) are obtained, see Table 1.27,29 Scanning electron microscopy pictures of CA and CA-5.1 after infiltration of NaAlH4 are compared in Fig. S1 in the ESI,‡ revealing a significant visible difference on the macroscopic scale since the CO2 activated sample appears more rough and granular. In contrast, the nano-pore size, Dmax, remained less affected in accordance with previous studies, see Table 1.27–29,34 The final morphology of activated aerogels strongly depends on the structure and composition of the starting material.35 The activated CA materials were less homogeneous than those prior to activation, which led to a broader distribution in the surface area (±270 m2 g−1) and total pore volume (±0.16), see Table 1. This may be due to thermal gradients and gas flow gradients during the activation process (see the ESI‡). Carbon aerogel CA-5.1 was functionalized with TiCl3 nanoparticles (sample CA–Ti) in order to study the combined effects of increased surface area and the addition of a catalyst.3.2. Melt infiltration of NaAlH4
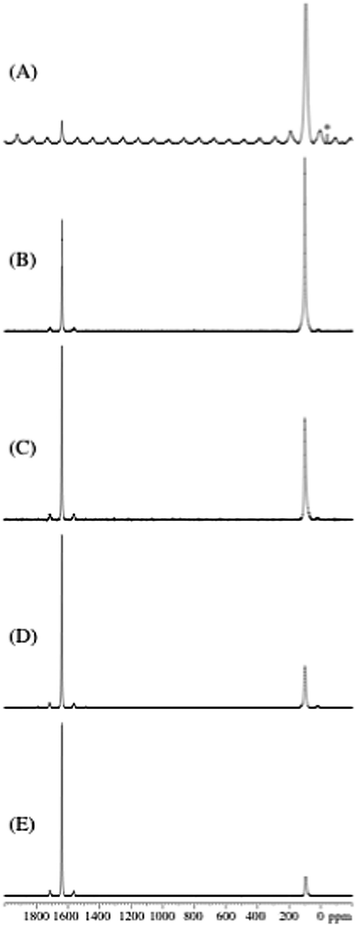
Sodium alanate, NaAlH4, was melt infiltrated to obtain nanoconfined samples with similar pore filling (∼60 vol%), see Table 1. Activated aerogels have an increased surface area and total pore volume and incorporate more NaAlH4 and therefore contain gravimetrically increasing amounts of hydride.The nanoconfined samples were investigated by 27Al MAS NMR in order to estimate the relative amounts of NaAlH4 and metallic Al, employing the same approach as used in earlier studies of a mixture of Al–LiAlH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36and of Al–NaAlH4.15 The 27Al MAS NMR spectrum (Fig. 1A) of NaAlH4 melted and cooled in the absence of a nanoscaffold (Na-m) shows centerband resonances from NaAlH4 (93.7 ppm) and metallic Al (1639.5 ppm) in accordance with the 27Al chemical shifts and quadrupolar couplings earlier reported for these compounds.16,37,38 In addition, spinning sidebands from the 27Al satellite transitions are observed for Na-m, indicating a higher degree of local structural order for NaAlH4 in this sample as compared to the nanoconfined infiltrated samples. Resonances from NaAlH4 and metallic Al only are also observed for the infiltrated samples (Fig. 1B–E) with relative signal intensities that are significantly different. It is observed that the intensity for metallic Al decreases for longer CO2 activation times. This corresponds to a more efficient melt infiltration of NaAlH4 in the CO2 activated CA scaffolds (Fig. 1B–E).
36and of Al–NaAlH4.15 The 27Al MAS NMR spectrum (Fig. 1A) of NaAlH4 melted and cooled in the absence of a nanoscaffold (Na-m) shows centerband resonances from NaAlH4 (93.7 ppm) and metallic Al (1639.5 ppm) in accordance with the 27Al chemical shifts and quadrupolar couplings earlier reported for these compounds.16,37,38 In addition, spinning sidebands from the 27Al satellite transitions are observed for Na-m, indicating a higher degree of local structural order for NaAlH4 in this sample as compared to the nanoconfined infiltrated samples. Resonances from NaAlH4 and metallic Al only are also observed for the infiltrated samples (Fig. 1B–E) with relative signal intensities that are significantly different. It is observed that the intensity for metallic Al decreases for longer CO2 activation times. This corresponds to a more efficient melt infiltration of NaAlH4 in the CO2 activated CA scaffolds (Fig. 1B–E).
The relative fractions of NaAlH4 and Al can be derived from the intensities in the 27Al MAS NMR spectra (Fig. 1), taking into account that the centerband resonance and first-order spinning sidebands for metallic Al correspond to the central as well as the satellite transition intensity whereas only the central transition is observed for NaAlH4 in the melt-infiltrated samples (Fig. 1B–E). These data are summarized in Table 2 and show that the amount of NaAlH4 infiltrated in the CA, CA-3, CA-5, CA-5.1 and CA–Ti samples increases with increasing surface area. The resonance from metallic Al is observed at 1639.5 ± 0.5 ppm for all samples and with very similar linewidths (FWHM = 3.3–3.7 ppm) for the melt infiltrated samples. Aluminium has a tendency to form larger nanoparticles outside the scaffold and may therefore interact more weakly with the scaffold.11,15 The frequency for the centerband resonance from NaAlH4 varies systematically for the infiltrated samples (Table 2) as illustrated in Fig. 2. The centerband resonance is shifted towards higher frequency and becomes more narrow (decreasing FWHM) with increasing degree of CO2 activation of the scaffolds (Fig. 2D–B). These variations may reflect differences in the interaction between nanoconfined NaAlH4 and the scaffold, potentially as a result of an increasing formation of graphite/graphene in the scaffold mediating electronic interactions between conduction electrons from graphite/graphene and the Al nucleus of the metal hydride.
| Sample | δ cg (ppm) | FWHM (ppm) | Alb (mol%) | NaAlH4b (mol%) | NaAlH4 crystallite size Lc (nm) | Alc (wt%) | NaAlH4c (wt%) | Nanoconf. fraction of NaAlH4c (wt%) |
|---|---|---|---|---|---|---|---|---|
| a Center of gravity for the centerband from the central transition. b Calculated from the 27Al MAS NMR spectra in Fig. 1, considering the fact that the resonances from metallic Al include both the central and satellite transitions whereas only the central-transition intensity is observed for NaAlH4. The estimated error limits are 2–4% for the relative molar intensities. c Calculated from Rietveld refinements of PXD data, Fig. 3. | ||||||||
| CA | 95.1 | 11.9 | 32 | 68 | 19 | 23 | 77 | 85 |
| CA-3 | 98.8 | 10.2 | 18 | 82 | 18 | 12 | 88 | 76 |
| CA-5 | 101.0 | 9.0 | 9 | 91 | 17 | 8 | 92 | 97 |
| CA-5.1 | 102.0 | 5.0 | 5 | 95 | 13 | 7 | 93 | 72 |
| CA–Ti | 100.9 | 6.2 | 4 | 96 | — | — | — | — |
| Na-m | 93.7 | 16.4 | 1 | 99 | — | — | — | — |
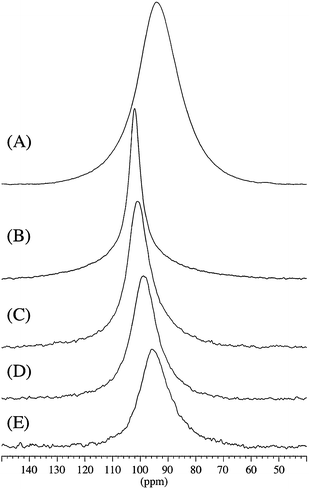 | ||
| Fig. 2 Expansion of the spectral region for the 27Al centerband resonances for NaAlH4 in the 27Al MAS NMR spectra shown in Fig. 1: (A) bulk NaAlH4 (Na-m), (B) melt infiltrated NaAlH4 in CA-5.1, (C) in CA-5, (D) in CA-3 and (E) in CA (not activated). | ||
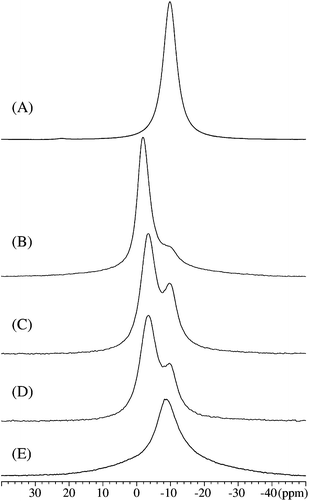
A similar effect is observed by 23Na MAS NMR, as illustrated in Fig. 3 for the same samples (measured after one year of storage at −40 °C). NaAlH4, bulk and confined in non-activated CA, gives a 23Na centerband resonance at −9.8 ppm (Fig. 3A and E). A new 23Na resonance is observed for NaAlH4 at higher frequency (−3.4 ppm to −1.9 ppm), which increases in intensity and exhibits a decreasing line width (FWHM = 5.8 ppm to 4.5 ppm) for longer CO2 activation times (Fig. 3D–B, respectively). This resonance is ascribed to electronic interactions between conduction electrons from an increasingly graphite/graphene-like scaffold and the Na nucleus of the metal hydride similar to the above mentioned observations by 27Al MAS NMR. A deconvolution for sample CA-5.1 (the spectrum shown in Fig. 3B) of the two partly overlapping centerband resonances reveals that 79.8% of NaAlH4 is infiltrated in activated CA whereas the remaining fraction is in less activated CA or is in a more bulk-like state. A high-frequency shift of the resonance from infiltrated NaAlH4 was also observed in a recent 23Na MAS NMR study of sodium alanate melt infiltrated in porous carbon.37,39 The observation of two distinct resonances (Fig. 3B–D) reflects that 23Na MAS NMR allows a more clear distinction of NaAlH4 infiltrated in activated and non-activated CA. In fact, the high-frequency shifted resonance for NaAlH4 infiltrated in activated CA observed in the 27Al MAS NMR spectra (Fig. 2B–D) has a low-frequency shoulder which gives the asymmetric lineshapes with a tail to low frequency for the centerband.
The improved resolution of the resonances from nanoconfined NaAlH4 in the 23Na MAS NMR spectra may result from several effects such as smaller quadrupole couplings for 23Na as compared to 27Al and a stronger interaction of the 23Na spins with the electrons of the scaffold material.
Sample Na-m contains only traces of Al (∼1 mol%), confirming that bulk NaAlH4 is stable towards the conditions used for melt infiltration, in agreement with the literature.2 Regardless hereof, molten NaAlH4 partially decomposes during melt infiltration into the scaffolds, as revealed from the presence of Al. However, CO2 activation and the higher surface area appear to stabilize molten NaAlH4 and a lower fraction decomposes to Al (see Fig. 1). This new effect may be explained by alterations in the aerogel morphology, e.g. larger fraction of micropores, higher surface area and pore volume, or in the chemical composition. Previous studies reveal that CO2 activation of RF-CAs increases the CA skeletal density towards that of pure graphite (e.g. ρRF-CA ∼ 1.89, ρRF-CA, activated ∼ 2.18 and ρgraphite = 2.25 g cm−3).27,35 Different carbon additives such as graphite, graphene, fullerene and carbon nanotubes have substantially different properties regarding catalysis of hydrogen release and uptake in NaAlH4.24,25 In that perspective, the observed stabilization of molten NaAlH4 may be explained by a graphitization of the activated aerogels. The infiltration process remains similarly efficient in the titanium functionalized activated RF-CA (sample CA–Ti incorporates 96 wt% of added NaAlH4).
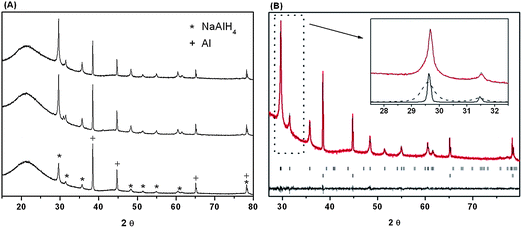
The amount of bulk NaAlH4 relative to nanoconfined NaAlH4 after the infiltration process was investigated by Rietveld refinement of powder X-ray diffraction data, shown in Fig. 4A. The analysis reveals that the Bragg diffraction peak shapes can be modeled as a superposition of a broad contribution from nano-confined NaAlH4 and a narrow contribution from bulk NaAlH4 (Fig. 4B). This approach provides the ratio of nanoconfined versus bulk NaAlH4 as well as the average particle size for nanoconfined NaAlH4 and the amount of Al present in the sample due to decomposition of NaAlH4, see Table 2. Only a minor fraction, 7 to 23 wt%, is decomposed to Al and a majority of the remaining NaAlH4, 72 to 97 wt%, is successfully nanoconfined, in accordance with the 23Na and 27Al MAS NMR data. The results also reveal decreasing average crystallite sizes of ∼19, 18, 17 and 13 nm for NaAlH4 nanoconfined in CA, CA-3, CA-5 and CA-5.1, respectively, which has maximum of the BET pore size distributions in the range of 8 to 11 nm (see Table 1). This observation indicates that NaAlH4 may preferably crystallize in the larger pores of the carbon aerogel.
The results obtained from 27Al and 23Na MAS NMR and powder X-ray diffraction for freshly prepared samples and samples stored for one year at −40 °C under argon are in accord and reveal high sample stability under the selected conditions. Minor discrepancies may be attributed to sample inhomogeneity.
3.3. Effects of surface area on the hydrogen release kinetics
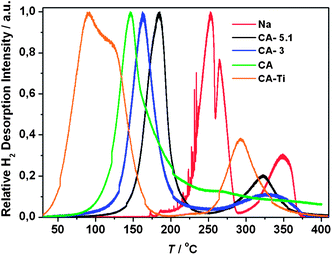
The first hydrogen desorption for all samples was investigated using simultaneous temperature programmed desorption and mass spectroscopy, TPD-MS (Fig. 5). The onset temperatures for hydrogen release, Tonset, and the temperatures at which the relative H2 desorption rate reach a maximum value, Tmax, are compared in Table 3. The onset temperatures for hydrogen release are clearly increasing with increasing CO2 activation of the CAs and the titanium functionalized scaffold reveals a Tonset, which is significantly lower and similar to a previous study using a Ti-functionalized non-activated CA.15The recorded Tmax values for NaAlH4 infiltrated in CA, CA-3, CA-5 and CA-5.1 are 147, 163, 180 and 184 °C, respectively, i.e. the hydrogen desorption rates clearly decrease with increasing CO2 activation of CA. Furthermore, Tmax values approach the melting point of NaAlH4 (Tmp = 183 °C) indicating that the observed stabilization of molten NaAlH4 during melt infiltration and nanoconfinement may be a kinetic effect, i.e. a result of the higher decomposition temperature. Thus, non-activated CA provides a higher degree of decomposition and agglomeration of Al particles outside the scaffold. The Tmax values for the activated samples remain significantly lower than that for bulk NaAlH4 (Na-m) of Tmax = 252 °C. However, CA–Ti incorporates 96 wt% of the added NaAlH4 and simultaneously has low Tmax values of 91 and 120 °C owing to titanium functionalization, see Tables 2 and 3.15 The two observed low temperature maxima on the TPD-MS curve for sample CA–Ti may be due to hydrogen release from NaAlH4 followed by Na3AlH6.
The TPD-MS profile of bulk NaAlH4 (Na-m without the aerogel) reveals local Tmax values at 252, 265 and 348 °C assigned to hydrogen release from NaAlH4, Na3AlH6 and NaH(s) forming Na(l). For nanoconfined samples the latter local maximum is usually less evident, e.g. NaAlH4 in CA, see Fig. 5.15,16 However, hydrogen release from NaH is clearly observed for the activated samples, NaAlH4 in CA-3, CA-5 and CA-5.1, with local Tmax values of 333, 313 and 326 °C, respectively, see Fig. 5 and S2 in the ESI.‡ This resembles the TPD-MS desorption profiles reported for NaAlH4 melt infiltrated onto the surface of non-porous graphite.16
Furthermore, comparing the kinetics for hydrogen release and uptake in NaAlH4 ball milled with different additives, the following trend is observed: RF-CA > activated carbons > graphite, graphene.25 Therefore, the increasing Tmax values for NaAlH4 and more intense observation of NaH by TPD-MS (Fig. 5) for the CO2-activated samples may be explained as an increasing graphitization of the scaffold.27 Noteworthy, there is limited variation in the apparent crystallite sizes of NaAlH4 in CA, CA-3, CA-5 and CA-5.1 within ∼13 to 19 nm, which is shown to have a limited effect on the kinetics.11
3.4. Stability during hydrogen exchange
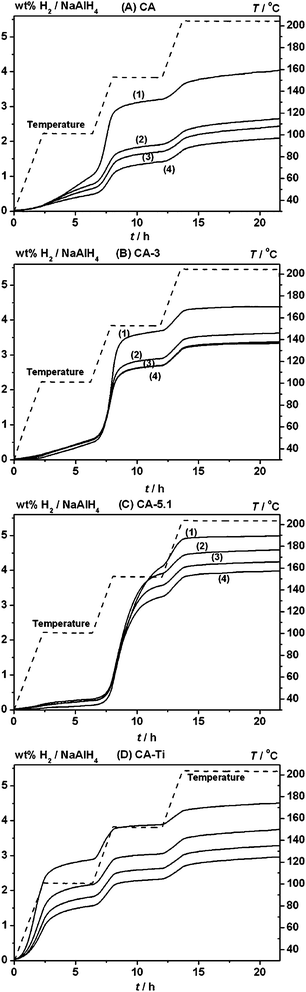
Sieverts' measurements were utilized to study the stability of the samples during four hydrogen release and uptake cycles, see Fig. 6. NaAlH4 nanoconfined in CA, CA-3 and CA-5.1 releases 1.3, 1.9, and 2.7 wt% H2 (relative to the mass of the sample), respectively, during the first desorption, corresponding to 72.1, 78.1 and 89.1% of the calculated hydrogen release relative to the used amount of NaAlH4, see Table 4. The results reveal that a higher fraction of NaAlH4 is successfully infiltrated in the CO2 activated scaffolds without decomposing, in agreement with 23Na/27Al MAS NMR and PXD data, see Fig. 1–4.| Sample | ρ m(H2) wt% H2/samplea | 1.Des/sample wt% H2b | 1.Des/NaAlH4 wt% H2 | 2.Des/NaAlH4 wt% H2 | 3.Des/NaAlH4 wt% H2 | 4.Des/NaAlH4 wt% H2 |
|---|---|---|---|---|---|---|
| a Calculated based on the amount of added NaAlH4, considering the purity of 90%. b Calculated from the H2 release measured by Sieverts' data. c Sieverts' data are shown in the ESI, Fig. S4. | ||||||
| CA | 1.8 | 1.3 (72.1) | 4.0 (100) | 2.7 (65.7) | 2.4 (60.3) | 2.1 (51.9) |
| CA-3 | 2.2 | 1.9 (78.1) | 4.4 (100) | 3.6 (83.0) | 3.4 (77.3) | 3.3 (76.2) |
| CA-5c | 2.7 | 2.4 (89.2) | 5.0 (100) | 4.3 (85.7) | 3.8 (76.9) | 3.7 (74.2) |
| CA-5.1 | 3.0 | 2.7 (89.1) | 5.0 (100) | 4.6 (91.9) | 4.2 (85.1) | 4.0 (79.8) |
| CA–Ti | 2.6 | 2.1 (80.4) | 4.5 (100) | 3.8 (83.4) | 3.3 (73.0) | 3.0 (65.8) |
Sodium alanate in CA releases 4.0, 2.7, 2.4 and 2.1 wt% H2/NaAlH4 during the first, second, third, and fourth desorption cycles, i.e. 100.0, 65.7, 60.3 and 51.9% of the initial H2 content, respectively, showing that nanoconfinement of NaAlH4 in non-activated RF-CAs suffers from significant loss of hydrogen storage capacity in agreement with previous studies.15 In contrast, NaAlH4 in CA-3 releases 83.0, 77.3 and 76.2% and in CA-5.1 91.9, 85.1 and 79.8% of the initial hydrogen content during desorption cycles 2, 3 and 4, respectively. Thus, the activation of RF-CAs significantly improves the stability and reversible hydrogen storage capacity of nanoconfined NaAlH4. This is a surprising result, suggesting that more graphite-like scaffolds tend to facilitate the infiltration process, stabilize nanoconfined NaAlH4 and preserve a higher hydrogen storage capacity over several cycles of hydrogen release and uptake. NaAlH4 infiltrated in activated and functionalized CA–Ti scaffolds releases 2.1 wt% H2/sample during the first desorption corresponding to 4.5 wt% H2/NaAlH4. Interestingly, a total of 2.90 wt% H2/NaAlH4 is released at T ≤ 100 °C and this amount corresponds to 64% of the total hydrogen content. During decomposition of bulk NaAlH4, 50% of the hydrogen content is released during the initial step forming Na3AlH6 and 25% of the hydrogen is released during the second step when Na3AlH6 decomposes to NaH and Al. Therefore, the current results indicate full decomposition of NaAlH4 at T ≤ 100 °C. NaAlH4 in CA–Ti releases 83.4, 73.0 and 65.8% of the initial hydrogen content during desorption cycles 2, 3 and 4, respectively, see Table 4 and Fig. 5D. Noteworthy, NaAlH4 in CA–Ti has intermediate cyclic properties, i.e. it is more stable than the non-activated samples but less stable than the activated sample.
This study demonstrates that activated carbon aerogels have increased surface area and pore volume and confine more NaAlH4 than non-activated CA. In addition, molten NaAlH4 appears to be stabilized by the higher surface area and possibly more graphite/graphene-like scaffolds, which provide a more efficient melt infiltration process. Furthermore, the stability of nanoconfined NaAlH4 during continuous hydrogen release and uptake is remarkably improved, i.e. CO2 activation of aerogels is an efficient method to significantly increase and preserve the hydrogen storage capacity of nanoconfined NaAlH4. Furthermore, considering absolute (100%) pore filling of a CO2-activated aerogel, e.g. CA-5.1 with Vtot = 2.38 cm3 g−1, has the potential to incorporate 68 wt% NaAlH4 corresponding to 3.4 wt% H2/sample.
The hydrogen desorption kinetics decrease with increasing surface area (Fig. 5 and 6). However, this study clearly demonstrates that activated aerogels can be functionalized with TiCl3 in order to combine the prolific properties from nanoconfinement, the efficient melt infiltration and the catalytic effect from titanium facilitating fast hydrogen release below 100 °C, see Fig. 5 and 6D. The surface area and TiCl3 content may be further optimized for the functionalized scaffolds in order to prepare ultimate materials of nanoconfined NaAlH4. The activated carbon aerogels may have similar prolific properties for nanoconfinement of other metal hydrides such as LiBH4, Mg(BH4)2 and MgH2 and may attract significant attention in the field of nanoconfinement. The mobility of hydrogen atoms on the surface of graphene and graphite is high and comparable to hydrogen atoms in the gas phase.22
4. Conclusions
This work reveals that CO2-activated carbon aerogels (CAs) have increased surface area and pore volume and incorporate more nanoconfined NaAlH4. The NaAlH4 melt infiltration process was significantly improved for the CO2 activated CA, which incorporates up to 91 mol% of the added NaAlH4 in contrast to only 52 mol% nanoconfined in as prepared CA. The available hydrogen content was increased from 1.3 wt% H2/sample in CA to 2.7 wt% H2/sample in the activated CA using ∼60 vol% pore filling. Considering 100% pore filling, a CO2-activated aerogel may reach a hydrogen storage capacity of 3.4 wt% H2/sample. Furthermore, the stability of nanoconfined NaAlH4 over several cycles of hydrogen release and uptake was significantly improved for the CO2 activated scaffolds. These new prolific effects may originate from a graphitization and/or chemical inertness of the CO2-activated scaffolds. In contrast, the desorption kinetics for hydrogen release from NaAlH4 decrease due to nanoconfinement in the CO2-activated aerogels but remain superior compared to bulk NaAlH4. This drawback is solved by TiCl3 nano-particle functionalization of RF-CA scaffolds. NaAlH4 efficiently melt infiltrates such Ti-functionalized scaffolds and shows fast H2 desorption kinetics at T < 100 °C. Activated RF-aerogels are relatively easy to prepare, appear to be more chemically inert and may be utilized to optimize nanoconfined LiBH4, Mg(BH4)2, MgH2 and other metal hydrides.Acknowledgements
The work was supported by CarlsbergFondet, the Danish National Research Foundation, Centre for Materials Crystallography (DNRF93), the Danish Strategic Research Council (Centre for Energy Materials and the project HyFillFast) and the Danish Council for Independent Research Natural Science (DanScatt). The access to beam time at the MAX-II synchrotron, Lund, Sweden in the research laboratory MAX-lab is gratefully acknowledged. The use of the facilities at the Instrument Centre for Solid-State NMR Spectroscopy, Aarhus University, sponsored by the Danish Council for Independent Research: Natural Sciences, the Danish Technical Science Research Council, CarlsbergFondet and Director Ib Henriksens Foundation, is acknowledged. We are grateful to the Polish Ministry of Science and Higher Education (Key Project POIG.01.03.01-14-016/08 and POIG.02.01.00-14-071/08/00).References
- D. J. C. Mackay, Sustainable Energy – Without the Hot Air, UIT Cambridge Ltd., England, 2009 Search PubMed.
- B. Bogdanovic, R. A. Brand, A. Marjanovic, M. Schwickardi and J. Tölle, J. Alloys Compd., 2000, 302, 36–58 CrossRef CAS.
- M. Dornheim, N. Eigen, G. Barkhordarian, T. Klassen and R. Bormann, Adv. Eng. Mater., 2006, 8, 377–385 CrossRef CAS.
- J. Graetz, Chem. Soc. Rev., 2009, 38, 73–82 RSC.
- L. H. Rude, T. K. Nielsen, D. B. Ravnsbaek, U. Bösenberg, M. B. Ley, B. Richter, L. M. Arnbjerg, M. Dornheim, Y. Filinchuk and F. Besenbacher, et al. , Phys. Status Solidi A, 2011, 208, 1754–1773 CrossRef CAS.
- U. Eberle, M. Felderhoff and F. Schüth, Angew. Chem., Int. Ed., 2009, 48, 6608–6630 CrossRef CAS PubMed.
- P. E. de Jongh and P. Adelhelm, ChemSusChem, 2010, 3, 1332–1348 CrossRef CAS PubMed.
- J. J. Vajo, Curr. Opin. Solid State Mater. Sci., 2011, 15, 52–61 CrossRef CAS PubMed.
- T. K. Nielsen, F. Besenbacher and T. R. Jensen, Nanoscale, 2011, 3, 2086–2098 RSC.
- M. Fichtner, Phys. Chem. Chem. Phys., 2011, 13, 21186–21195 RSC.
- T. K. Nielsen, P. Javadian, M. Polanski, F. Besenbacher, J. Bystrzycki and T. R. Jensen, J. Phys. Chem. C, 2012, 116, 21046–21051 CAS.
- Z. Zhao-Karger, J. Hu, A. Roth, D. Wang, C. Kubel, W. Lohstroh and M. Fichtner, Chem. Commun., 2010, 46, 8353–8355 RSC.
- T. K. Nielsen, K. Manickam, M. Hirscher, F. Besenbacher and T. R. Jensen, ACS Nano, 2009, 3, 3521–3528 CrossRef CAS PubMed.
- A. F. Gross, J. J. Vajo, S. L. Van Atta and G. L. Olson, J. Phys. Chem. C, 2008, 112, 5651–5657 CAS.
- T. K. Nielsen, M. Polanski, D. Zasada, P. Javadian, F. Besenbacher, J. Bystrzycki, J. Skibsted and T. R. Jensen, ACS Nano, 2011, 5, 4056–4064 CrossRef CAS PubMed.
- J. Gao, P. Adelhelm, M. H. W. Verkuijlen, C. Rongeat, M. Herrich, P. J. M. van Bentum, O. Gutfleisch, A. P. M. Kentgens, K. P. de Jong and P. E. de Jongh, J. Phys. Chem. C, 2010, 114, 4675–4682 CAS.
- R. D. Stephens, A. F. Gross, S. L. Van Atta, J. J. Vajo and F. E. Pinkerton, Nanotechnology, 2009, 20, 204018 CrossRef PubMed.
- M. Paskevicius, D. A. Sheppard and C. E. Buckley, J. Am. Chem. Soc., 2010, 132, 5077–5083 CrossRef CAS PubMed.
- Z. Zhao-Karger, R. Witter, E. G. Bardaji, D. Wang, D. Cossement and M. Fichtner, J. Mater. Chem. A, 2013, 1, 3379–3386 CAS.
- A. Remhof, P. Mauron, A. Zuttel, J. P. Embs, Z. Lodziana, A. J. Ramirez-Cuesta, P. Ngene and P. de Jongh, J. Phys. Chem. C, 2013, 117, 3789–3798 CAS.
- M. Paskevicius, H. Y. Tian, D. A. Sheppard, C. J. Webb, M. P. Pitt, E. M. Gray, N. M. Kirby and C. E. Buckley, J. Phys. Chem. C, 2011, 115, 1757–1766 CAS.
- H. M. Cuppen and L. Hornekaer, J. Chem. Phys., 2008, 128, 174707 CrossRef CAS PubMed.
- B. Bogdanovic and M. Schwickardi, J. Alloys Compd., 1997, 253–254, 1–9 CrossRef CAS.
- P. A. Berseth, A. G. Harter, R. Zidan, A. Blomqvist, C. M. Araújo, R. H. Scheicher, R. Ahuja and P. Jena, Nano Lett., 2009, 9, 1501–1505 CrossRef CAS PubMed.
- F. E. Pinkerton, J. Alloys Compd., 2011, 509, 8958–8964 CrossRef CAS PubMed.
- T. K. Nielsen, U. Bösenberg, R. Gosalawit, M. Dornheim, Y. Cerenius, F. Besenbacher and T. R. Jensen, ACS Nano, 2010, 4, 3903–3908 CrossRef CAS PubMed.
- C. Lin and J. A. Ritter, Carbon, 2000, 38, 849–861 CrossRef CAS.
- T. F. Baumann, M. A. Worsley, T. Y. J. Han and J. H. Satcher, J. Non-Cryst. Solids, 2008, 354, 3513–3515 CrossRef CAS PubMed.
- S. A. Al-Muhtaseb and J. A. Ritter, Adv. Mater., 2003, 15, 101–114 CrossRef CAS.
- J. H. de Boer, B. G. Linsen, T. van der Plas and G. J. Zondervan, J. Catal., 1965, 4, 649–653 CrossRef CAS.
- S. Brunauer, P. H. Emmett and E. Teller, J. Am. Chem. Soc., 1938, 60, 309–319 CrossRef CAS.
- E. P. Barrett, L. G. Joyner and P. P. Halenda, J. Am. Chem. Soc., 1951, 73, 373–380 CrossRef CAS.
- J. Rodriguez-Carvajal, FULLPROF SUITE, 2003 Search PubMed.
- Y. Hanzawa, K. Kaneko, R. W. Pekala and M. S. Dresselhaus, Langmuir, 1996, 12, 6167–6169 CrossRef CAS.
- M. S. Contreras, C. A. Paez, L. Zubizarreta, A. Leonard, S. Blather, C. G. Olivera-Fuentes, A. Arenillas, J. P. Pirard and N. Job, Carbon, 2010, 48, 3157–3168 CrossRef CAS PubMed.
- L. Kellberg, H. Bildsøe and H. J. Jakobsen, J. Chem. Soc., Chem. Commun., 1990, 1294–1295 RSC.
- M. H. W. Verkuijlen, J. Gao, P. Adelhelm, P. J. M. van Bentum, P. E. de Jongh and A. P. M. Kentgens, J. Phys. Chem. C, 2010, 114, 4683–4692 CAS.
- B. Bogdanovic, M. Felderhoff, M. Germann, M. Hartel, A. Pommerin, F. Schuth, C. Weidenthaler and B. Zibrowius, J. Alloys Compd., 2003, 350, 246–255 CrossRef CAS.
- P. Adelhelm, J. B. Gao, M. H. W. Verkuijlen, C. Rongeat, M. Herrich, P. J. M. van Bentum, O. Gutfleisch, A. P. M. Kentgens, K. P. de Jong and P. E. de Jongh, Chem. Mater., 2010, 22, 2233–2238 CrossRef CAS.
Footnotes |
| † Dedicated to the memory of a colleague, friend and mentor professor Jerzy Bystrzycki who suddenly passed away much too early. |
| ‡ Electronic supplementary information (ESI) available. See DOI: 10.1039/c3nr03538g |
| This journal is © The Royal Society of Chemistry 2014 |