Effect of 1D twisted water chains confined in channels formed by a Gemini amphiphile on its crystal stability†
Abstract
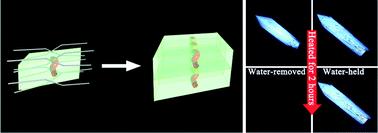
A novel gemini amphiphile bearing a rigid spacer was found to form inclusion crystals with water in 1 : 3 host-to-guest molar ratios. The host molecules can generate a hydrophobic cavity unit via intermolecular intersections between each two neighbouring molecules, which can be connected with one another in one direction, finally resulting in a channel. At the same time, one-dimensional (1D) water chains are trapped in the channels by weak C–H⋯O, O–H⋯Br− and C–H⋯Br− interactions with the walls composed of the host molecules. These interactions and water chains together with the alternating water chain and host molecule layers can form a two-dimensional network in the crystal, which can stabilise the crystal structure. The profound contribution of the water chains to the stability of the host framework is verified by comparisons of properties between water-containing crystals and anhydrous materials. Meanwhile, weak interactions are theoretically calculated and visualised by non-covalent interaction (NCI) analysis and further confirmed by spectral experiments. Both experiment and calculation reveal that these weak hydrogen bonds provide cohesion that is vital to crystal stability.

 Please wait while we load your content...
Please wait while we load your content...