Crystal disordering and organic solid-state reactions
Abstract
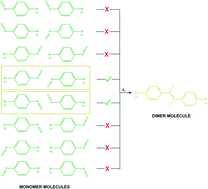
This work is the extension of our preliminary communication about the relationship between the change in the solid-state structure and the chemical reaction in a crystal. The investigation is a case study about the nature of the adiabatic organic solid-state reactions by kinetic measurements of the processes that occur during the dimerization of aromatic nitroso compounds (p-bromo- and p-iodonitrosobenzenes, respectively) in crystals. From the reaction rates at different temperatures we have calculated the activation energy. Since dimerization is induced by the appearance of crystal deformations on the surface caused by sublimation, we study here the relationship between the rate of sublimation and the rate of dimerization. There are indications that the sublimation of molecules from the surface serves only for the activation of chemical solid-state reaction. Dimerization is also studied under three different topochemical environments: strong topochemistry in the cryogenic conditions where the starting monomers were obtained by photolysis of crystals of the corresponding dimer at 14 K, looser topochemistry in the freshly sublimed crystals of monomers, and randomly distributed monomer molecules in thin layers prepared by cryogenic deposition. It is demonstrated that the change in topochemistry drastically modifies the reaction rate.

 Please wait while we load your content...
Please wait while we load your content...