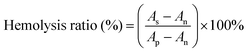Toward a highly hemocompatible membrane for blood purification via a physical blend of miscible comb-like amphiphilic copolymers†
Fen
Ran
*ab,
Xiaoqin
Niu
c,
Haiming
Song
b,
Chong (Sage)
Cheng
a,
Weifeng
Zhao
a,
Shengqiang
Nie
a,
Lingren
Wang
a,
Aimei
Yang
c,
Shudong
Sun
a and
Changsheng
Zhao
*a
aCollege of Polymer Science and Engineering, State Key Laboratory of Polymer Materials Engineering, Sichuan University, Chengdu 610065, People's Republic of China. E-mail: zhaochsh70@163.com; zhaochsh70@scu.edu.cn; Fax: +86-28-85405402; Tel: +86-28-85400453
bSchool of Material Science and Engineering, State Key Laboratory of Gansu Advanced Non-Ferrous Metal Materials, Lanzhou University of Technology, Lanzhou 730050, People's Republic of China. E-mail: ranfen@163.com
cCollege of Life Science and Engineering, Lanzhou University of Technology, Lanzhou 730050, People's Republic of China
First published on 8th January 2014
Abstract
Comb-like amphiphilic copolymers (CLACs) consisting of functional chains of poly(vinyl pyrrolidone) and polyethersulfone-based hydrophobic chains were firstly synthesized by reversible addition–fragmentation chain transfer polymerization. The CLAC can be used as an additive to blend with polyethersulfone (PES) at any ratio due to the excellent miscibility, and then a surface segregation layer with permanent hydrophilicity could be obtained. The surfaces of the CLAC modified PES membranes were characterized using X-ray photoelectron spectroscopic analysis, Fourier transform infrared and water contact angle measurements. The surfaces are self-assembled with numerous functional branch-like –PVP chains, which can improve the hemocompatibility. The root-like –PES chains (the hydrophobic part) are embedded in the membranes firmly, which greatly reduces the elution during the membrane preparation procedure and repeated usage, and makes the membranes have a permanent stability. The PES-based hydrophobic chains have the same structure as the membrane bulk material, which makes the miscibility of the additive and the membrane material good to ensure the intrinsic properties of the membrane. The modified membranes showed suppressed platelet adhesion and prolonged blood coagulation time (activated partial thromboplastin time, APTT); thus, the blood compatibility of the membranes was highly improved. The strategy may be extended to synthesize other PES-based functional copolymers and to prepare a modified PES dialysis membrane for blood purification.
1. Introduction
Current blood purification membranes for hemodialysis, hemodiafiltration, hemofiltration, plasmapheresis, and plasma collection have limitations since they may trigger thrombus formation.1 Therefore, hemocompatibility has been considered as the key to clinical success of blood contacted medical treatment.2 In the past few decades, with the aim of avoiding medical negligence or reducing anticoagulant dosage, tremendous efforts had been made to improve the hemocompatibility of biomaterials for biomedical applications.3 It is well-known that protein adsorption is the first event when blood contacts the membrane surface in a blood purification device, and then leads to platelet adhesion and even blood clotting.4 Therefore, many approaches were employed to decrease membrane protein adsorption by surface modification, such as introducing functional groups onto membrane surfaces.5Many functional molecules and biomacromolecules, such as endothelial cells,6 protein,7 heparin8 and phospholipid polymers,9 have been introduced onto polymeric membrane surfaces, and the modified membranes showed improved biocompatibility. However, none of them is ideal for clinical applications, due to high costs and technical problems during membrane preparation. Furthermore, many modification techniques, such as grafting, blending and coating methods, have been used to modify membrane surfaces to simulate the structures of these molecules and biomacromolecules.10 Chemical grafting is the most efficient method, while blending is the most widely used method in industry and can be used to modify a hollow fibre membrane.11 Directly blending functional additives containing hydrophilic,12 negatively charged,13 and/or heparin-like14 chains into a membrane matrix may endow the membrane surfaces with anticoagulant, antifouling, and antithrombotic properties. However, the elution of water soluble additives15 and the poor miscibility of the additives16 may be the challenges to reuse the modified membranes. For overcoming these problems, the most effective, but always difficult approach is constructing an amphiphilic additive using the membrane matrix macromolecules as the hydrophobic chains.
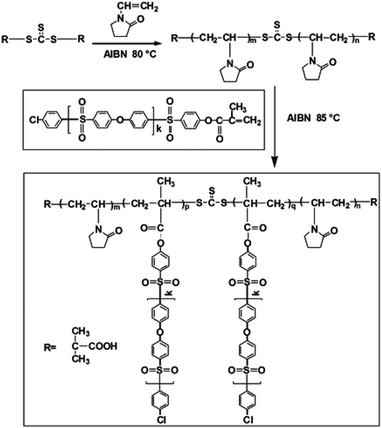
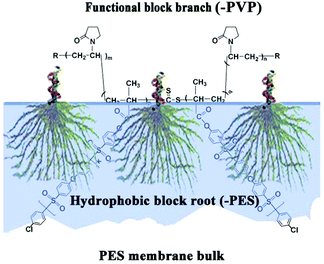
In this work, comb-like amphiphilic copolymers (CLACs) consisting of branch-like functional chains of poly(vinyl pyrrolidone) (–PVP) and polyethersulfone (PES)-based root-like hydrophobic chains (–PES) were firstly synthesized, and used to modify the PES membrane. The CLACs are miscible with PES, and can be used as additives to blend with PES at any ratio, and high hemocompatibility property will be obtained. Scheme 1 shows a schematic representation of the membrane surface segregation layer architecture by assembly of the CLAC. Why such a structured membrane shows a good comprehensive performance can be summarized as follows: Firstly, the surface is self-assembled with numerous functional branch-like –PVP chains, which can improve the hemocompatibility. Secondly, the root-like –PES chains (the hydrophobic part) are embedded in the membrane firmly, which greatly reduces the elution during the membrane preparation procedure and repeated usage, and makes the membrane have a permanent stability. Thirdly, the PES-based hydrophobic chains have the same structure as the membrane bulk material, which makes the miscibility of the additive and the membrane material good to ensure the intrinsic properties of the membrane.
 | ||
| Scheme 1 Schematic representation of membrane surface segregation layer architecture by the CLAC copolymer assembly. | ||
2. Materials and methods
2.1 Materials
PES (Ultrason E6020P, molecular weight is 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was obtained from BASF (Germany). Vinyl pyrrolidone (VP, 99.0%) was purchased from Alfa Aesar (USA), and was pre-treated with activated carbon before use. 4,4′-Dichlorodiphenylsulfone, methacryloyl chloride and benzyltriethylammonium chloride were purchased from Aladdin (Shanghai, China), and were used as received. Azo-bis-isobutryonitrile (AIBN), N,N-dimethylformamide (DMF) (99.0%), N,N-dimethylacetamide (DMAC) (99.0%), dichloromethane, dimethylsulfoxide (DMSO), sulfolane, and other chemicals were purchased from Kelong Inc. (Chengdu, China), and were used as received. S,S′-Bis(α,α′-dimethyl-α′′-acetic acid)-trithiocarbonate (reversible addition–fragmentation chain transfer agent, RAFT-agent) was synthesized according to a previous report.17 The preparation of platelet-poor plasma, the hemolysis test, and the clotting time test in these experiments were all performed by the Institute of Blood Transfusion (IBT), Chinese Academy of Medical Sciences. These experiments were all approved by the institutional committee and in compliance with the informed consent of the individual donating the samples. Also, all these experiments were performed in compliance with the relevant laws of the Chinese government and institutional guidelines.
000) was obtained from BASF (Germany). Vinyl pyrrolidone (VP, 99.0%) was purchased from Alfa Aesar (USA), and was pre-treated with activated carbon before use. 4,4′-Dichlorodiphenylsulfone, methacryloyl chloride and benzyltriethylammonium chloride were purchased from Aladdin (Shanghai, China), and were used as received. Azo-bis-isobutryonitrile (AIBN), N,N-dimethylformamide (DMF) (99.0%), N,N-dimethylacetamide (DMAC) (99.0%), dichloromethane, dimethylsulfoxide (DMSO), sulfolane, and other chemicals were purchased from Kelong Inc. (Chengdu, China), and were used as received. S,S′-Bis(α,α′-dimethyl-α′′-acetic acid)-trithiocarbonate (reversible addition–fragmentation chain transfer agent, RAFT-agent) was synthesized according to a previous report.17 The preparation of platelet-poor plasma, the hemolysis test, and the clotting time test in these experiments were all performed by the Institute of Blood Transfusion (IBT), Chinese Academy of Medical Sciences. These experiments were all approved by the institutional committee and in compliance with the informed consent of the individual donating the samples. Also, all these experiments were performed in compliance with the relevant laws of the Chinese government and institutional guidelines.
2.2 Synthesis of the macro-RAFT agent (poly(vinyl pyrrolidone), PVP)
The synthesis of the copolymer and the preparation of the modified membrane involve many steps and chemicals. In order to express them more clearly, many abbreviations are used: O, PES-MM, CLAC, and M represent the synthesized oligomer, the PES monovinylmacromolecular monomer, the comb-like amphiphilic copolymer, and the copolymer modified membrane, respectively.RAFT polymerization of VP was carried out in a sealed tube. The general procedure was as follows: VP (1.11 g, 11.10 mmol), RAFT agent (0.03 g, 0.11 mmol), AIBN (0.01 g, 0.06 mmol) and DMF (5 mL) were added to a tube; after bubbling with nitrogen for 30 min, the polymerization was carried out under a nitrogen atmosphere at 80 °C for 5 h. After precipitating the mixture in ethyl ether, the product was dried under vacuum at 50 °C overnight. The prepared carboxyl-terminated PVP was termed as a macro-RAFT agent for further RAFT polymerization.
2.3 Synthesis of PES monovinylmacromolecular monomer (PES-MM)
The preparation process of PES-MM mainly involves the synthesis of 4-chloro,4-hydroxydiphenylsulfone (CHDS), diphenylsulfone-mono-potassium salt (DSP), oligomer and PES-MM in turn. Scheme S1 in the ESI† shows the synthesis procedure of PES-MM.2.4 Synthesis of CLAC
The synthesis of CLAC was as follows: monomer (PES-MM-2, 0.5 g), AIBN (0.01 g), macro-RAFT agent (PVP, 0.3 g) and DMF (10 mL) were added in a sealed tube. The mixture was deoxygenated by nitrogen bubbling for 30 min, and the polymerization was carried out at 85 °C for 7 h. The final polymer was recovered by precipitating the mixture in diethyl ether. To remove the homopolymer of PVP and the copolymer with the short hydrophobic chain, the product was extracted in a Soxhlet extractor using water as the solvent for 1 week, and then dried under vacuum until a constant weight was achieved. CLACs prepared using PES-MM-1, PES-MM-2 and PES-MM-3 as the macromonomers were termed CLAC-1, CLAC-2 and CLAC-3, respectively.2.5 Polymer characterization
Fourier transform infrared (FTIR) spectra for the polymer and copolymer were measured using a FTIR Nicolet560 (Nicol America) instrument. To prepare FTIR samples, the polymer was dissolved in DMAC and casted on a potassium bromide disk with a thickness of about 0.8 mm. 1H-NMR spectra were recorded on a Varian Unity Plus 300/54 NMR spectrometer using DMSO-d6 as the solvent at 25 °C.The molecular weights of the oligomers were determined by end-group titration. 1 g of the reaction mixture was added to 20 mL of DMSO and was stirred for several minutes. The direct titration of the hydroxyl end-group was carried out using aqueous standard HCl solution as the titrant and using bromocresol green as the indicator. The weight average molecular weight of PVP was determined with SEC measurements provided with a light scattering detector (BI-200SM, Brookhaven Instruments Co., Holtsville, NY) using H2O as the solvent at 25 °C.
2.6 Membrane fabrication
PES/copolymer membranes were prepared by a phase inversion technique. PES and the synthesized copolymers were dissolved in DMAC by vigorous stirring until a clear homogeneous solution was obtained. The total concentration of the polymers was about 18.8 wt% (the PES/CLAC ratio was 1.6/0.8 (wt%/wt%)). After vacuum degassing, the casting solutions were prepared into membranes by spin coating coupled with a liquid–liquid phase separation technique at room temperature. The membranes prepared using CLAC-1, CLAC-2 and CLAC-3 as the additives were termed M-1, M-2 and M-3, respectively. The spin coating conditions were: rotation speed (1000–1500 rpm), rotation time (12–18 s) and at room temperature, and water was the non-solvent. The prepared membrane has a uniform thickness of about 60–70 μm, and a glass slide was used as the substrate. The spin-coated membrane separated from the substrate automatically and easily when they were immersed in distilled water. The membranes were thoroughly rinsed with physiological buffer solution (PBS) at 37 °C for 30 days to remove the residual solvent.In the experiments, different membranes were prepared by changing the weight percentage of the copolymer in the casting solutions. The amounts of the copolymer CLAC-2 in the casting solutions were 0, 1.11, 2.97, 4.50, 6.29, 8.12 and 9.47 wt%, respectively. The membranes with the PES/CLAC-2 (wt%/wt%) ratios of 1.6/0, 1.6/0.1, 1.6/0.3, 1.6/0.5, 1.6/0.8, 1.6/1.2 and 1.6/1.6 were termed M-PES, M-2-1, M-2-2, M-2-3, M-2-4, M-2-5 and M-2-6, respectively. The temperature of the coagulation bath was 25 °C. The compositions of the casting solutions are listed in Table 1.
| Samplea | Polymerb (18.8 wt%) | Solvent | ||
|---|---|---|---|---|
| Additive | ||||
| PES (g) | CLAC | Amount (g) | DMAC (g) | |
| a The casting solutions were uniform and transparent. b Solid contents of the polymers. | ||||
| M-PES | 1.6 | — | — | 7.0 |
| M-1 | 1.6 | CLAC-1 | 0.8 | 10.3 |
| M-2 | 1.6 | CLAC-2 | 0.8 | 10.3 |
| M-3 | 1.6 | CLAC-3 | 0.8 | 10.3 |
| M-2-1 | 1.6 | CLAC-2 | 0.1 | 7.3 |
| M-2-2 | 1.6 | CLAC-2 | 0.3 | 8.2 |
| M-2-3 | 1.6 | CLAC-2 | 0.5 | 9.0 |
| M-2-4 | 1.6 | CLAC-2 | 0.8 | 10.3 |
| M-2-5 | 1.6 | CLAC-2 | 1.2 | 12.0 |
| M-2-6 | 1.6 | CLAC-2 | 1.6 | 13.7 |
2.7 Membrane characterization
The morphologies of the membranes were observed by scanning electron microscopy (SEM) using an XL 30ESME scanning microscope. The membranes were frozen in liquid nitrogen, and then broken and sputtered with a gold layer before SEM observation. The structures and elements of the membrane surfaces were investigated by reflected FTIR and X-ray photoelectron spectroscopic analysis (XPS).The hydrophilicity of the membrane surface was characterized on the basis of contact angle measurements using a contact angle goniometer (OCA20, Dataphysics, Germany) equipped with a video capture. A piece of membrane 2 × 2 cm2 was attached to a glass slide and mounted in the goniometer. For the static contact angle (SCA) measurements, 3 μL double distilled water was dropped onto the surface of the membrane at room temperature and the contact angle was measured after 10 s. At least eight measurements were averaged to obtain a reliable value. The measurement error was ±3°.
2.8 Platelet adhesion
Healthy human fresh blood (man, 34 years old) was collected using vacuum tubes, containing sodium citrate (3.8 wt%) as an anticoagulant, and the ratio of the anticoagulant to blood was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 (V/V). The blood was centrifuged at 1500 rpm for 15 min to obtain platelet-rich plasma (PRP) or at 4000 rpm for 15 min to obtain platelet-poor plasma (PPP).
9 (V/V). The blood was centrifuged at 1500 rpm for 15 min to obtain platelet-rich plasma (PRP) or at 4000 rpm for 15 min to obtain platelet-poor plasma (PPP).
The PES and modified PES membranes were immersed in PBS solution and equilibrated at 37 °C for 1 h, and then the PBS solution was removed followed by introducing 1 mL of fresh PRP. The membranes were incubated with PRP at 37 °C for 2 h and the PRP was decanted. The membranes were rinsed 3 times with PBS solution. Finally, the membranes were treated with 2.5 wt% glutaraldehyde in PBS at 4 °C for 1 day. The samples were washed with PBS solution, and subjected to a drying process by passing them through a series of graded alcohol–PBS solutions (25, 50, 75 and 100 wt%) and isoamyl acetate–alcohol solutions (25, 50, 75 and 100 wt%). The platelet adhesion was observed using an S-2500C microscope (Hitachi, Japan).
2.9 Clotting time
To evaluate the antithrombogenicity of the membranes, activated partial thromboplastin time (APTT) was measured by an automated blood coagulation analyzer CA-50 (Sysmex Corporation, Kobe, Japan). The APTT was measured as follows: the samples (1 × 1 cm2, one piece) were incubated with platelet-poor plasma (0.15 mL) at 37 °C for 30 min, and then the APTT was measured.2.10 Hemolysis test
For the hemolytic test, sodium citrate stabilized human blood was obtained from Huaxi hospital firstly. 20 mL whole blood was centrifuged at 500g for 15 min to get concentrated red cell suspension. The suspension was washed with normal saline (NS) 5 times. The concentrated red cell suspension was diluted in NS and centrifuged at 500g for 15 min, and then the supernatant was removed. The suspension was diluted in NS with a final volume of 100 mL red blood cell (RBC) suspension. The sterilized membrane (1 × 1 cm2) was immersed in NS for 24 h, and then incubated in 1 mL diluted RBC suspension and incubated at 37 °C for 3 h. RBC suspended in deionized water was used as a positive control, and the NS-dispersed RBC suspension was used as a negative control. After incubation, the RBC suspensions were centrifuged at 500g for 5 min. The absorbance of the released hemoglobin in the suspensions was recorded by spectrophotometry at 546 nm. The hemolysis ratio was calculated using the following formula:where As, Ap and An are the absorbance of the sample suspension, positive control and negative control, respectively. Two parallel tests were carried out independently to get a reliable value.
2.11 Ultrafiltration of PEG solution
The permeability of polyethylene glycol (PEG-10000) was investigated in order to evaluate the membrane permeability. The feed solution was prepared by dissolving PEG-10000 in double distilled water with a concentration of 0.1 g L−1. The PEG-10000 solution was applied to measure the membrane ultrafiltration properties using the apparatus described in our earlier study.18 The concentration of PEG was determined using a UV-VIS spectrophotometer (Model 756, Shanghai Spectrophotometer Instrument Co., Ltd, China) at a wavelength of 510 nm after the reaction with BaCl2 and I2 solutions.19 The observed sieving coefficient (So) of PEG was defined as So = Cp/Cb, where Cp and Cb are the PEG concentrations in the permeate and bulk solutions, respectively.3. Results and discussion
3.1 Synthesis and characterization of CLAC
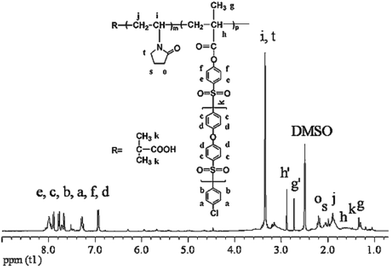
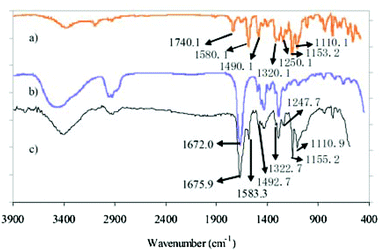
CLACs were conveniently synthesized by RAFT polymerization as shown in Scheme 2. The PES-MM was designed to have the end group required for the copolymerization. The preparation process of PES-MM mainly involves the synthesis of diphenylsulfone-mono-sylvite, oligomer and PES-MM in turn. For further preparing the copolymers, the synthesized PVP acted as a macro-RAFT agent and the reaction was conducted at 85 °C. To remove the homopolymer of PVP and the copolymer with short hydrophobic chains, the product was extracted in a Soxhlet extractor using water as the solvent for 1 week. Through the facile polymerization and copolymerization, the prepared copolymer possessed amphiphilic block structure and comb-like structure, which contained the backbone chain polymethacrylate (–PMA), the branch-like functional chain –PVP and the root-like hydrophilic chain –PES.The structure of the synthesized CLAC was confirmed by 1H-NMR, as shown in Fig. 1. The 1H-NMR spectrum of the CLAC recorded in DMSO-d6 at 25 °C revealed the presence of the characteristic signals of the –PES chain (6.85–8.18 ppm), the –PMA backbone (1.22–1.27 ppm) and the –PVP chain (3.00–3.51 ppm). The structure of the CLAC was further confirmed by the FTIR spectrum. As shown in Fig. 2, the strong characteristic peaks could be assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond (1675.9 cm−1), the C
O bond (1675.9 cm−1), the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond (1583.3 and 1492.7 cm−1), the O–Ar bond (1247.7 cm−1), and the asymmetric and symmetric stretching vibration of the O
C bond (1583.3 and 1492.7 cm−1), the O–Ar bond (1247.7 cm−1), and the asymmetric and symmetric stretching vibration of the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond (1155.2 and 1110.9 cm−1), respectively. It should be pointed out that the characteristic peak at 1675.9 cm−1 was an overlapped peak for the C
O bond (1155.2 and 1110.9 cm−1), respectively. It should be pointed out that the characteristic peak at 1675.9 cm−1 was an overlapped peak for the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching in the –PVP chain and the –PES chain. In fact, the C
O stretching in the –PVP chain and the –PES chain. In fact, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching peaks for PVP and PES-MM could be observed at 1740.1 and 1672.0 cm−1, respectively. This phenomenon can be ascribed to the strong donor–acceptor interaction between the O
O stretching peaks for PVP and PES-MM could be observed at 1740.1 and 1672.0 cm−1, respectively. This phenomenon can be ascribed to the strong donor–acceptor interaction between the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N group of the –PVP chain and the O
C–N group of the –PVP chain and the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and/or benzene ring of the –PES chain in the copolymer CLAC.20 In addition, the interaction between –PVP and –PES chains in the CLAC made the characteristic peaks of CLAC migrate. In a word, 1H-NMR and FTIR spectroscopic analysis indicated that the copolymer CLAC was successfully synthesized.
O and/or benzene ring of the –PES chain in the copolymer CLAC.20 In addition, the interaction between –PVP and –PES chains in the CLAC made the characteristic peaks of CLAC migrate. In a word, 1H-NMR and FTIR spectroscopic analysis indicated that the copolymer CLAC was successfully synthesized.
The average molecular weight and average copolymer composition of the copolymers were determined by combining the multi-angle light scattering detector, end-group titration and 1H-NMR. The weight average molecular weight (Mw) of the functional chain –PVP (the macro-RAFT agent PVP) was determined by SEC measurements using H2O as the solvent at 25 °C, and it was 6.9 × 103.21 In the preparation process, the molecular weight of the root-like hydrophobic chain –PES (that is, oligomer) was determined by end-group titration (see the Experimental section) and really depended on the reaction time. The molecular weight of PES-MM was calculated by the given molar ratio of the oligomer to methacryloyl chloride (molar ratio of the oligomer to methacryloyl chloride was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The molar ratio of PES-MM to VP in CLACs was determined by comparing the signals at δ = 6.85–8.18 ppm, δ = 3.01–3.51 ppm and δ = 1.25 ppm in the 1H-NMR spectrum. The results revealed that the composition and molecular weight of the copolymer could be controlled by changing the feed ratios. The composition and molecular weight data of the CLAC copolymers are summarized in Table 2, which indicate that the CLAC had similar –PVP chain length and –PMA backbone length, but different –PES chain lengths.
1). The molar ratio of PES-MM to VP in CLACs was determined by comparing the signals at δ = 6.85–8.18 ppm, δ = 3.01–3.51 ppm and δ = 1.25 ppm in the 1H-NMR spectrum. The results revealed that the composition and molecular weight of the copolymer could be controlled by changing the feed ratios. The composition and molecular weight data of the CLAC copolymers are summarized in Table 2, which indicate that the CLAC had similar –PVP chain length and –PMA backbone length, but different –PES chain lengths.
| Sample | M w of PVPa | M n of PES-MMb | Average copolymer compositionc |
|---|---|---|---|
| a Determined by a multi-angle light scattering detector using H2O as the solvent at 25 °C. b Determined by end-group titration. c The molar ratios of PES-MM to -PVP in CLACs were determined by 1H-NMR. | |||
| CLAC-1 | 6958 | 980 | PVP32-(PES4-MM)25-PVP32 |
| CLAC-2 | 6958 | 4692 | PVP32-(PES20-MM)23-PVP32 |
| CLAC-3 | 6958 | 6084 | PVP32-(PES27-MM)22-PVP32 |
3.2 Preparation and characterization of membranes
CLAC modified PES membranes were prepared by a liquid–liquid phase separation technique at room temperature. In order to investigate the elution of the copolymer, the membrane was immersed in distilled water at 37 °C for 30 days. The concentration of the eluted copolymer was tested using a UV-Vis spectrophotometer (UV-1750, Shimadzu Co., Ltd, Japan) at a wavelength of 190 to 600 nm, and it was less than the percent of the copolymer added (Fig. S1 in the ESI†). The results indicated that the amount of the eluted copolymer was very small. Fig. 3 shows the cross-sectional SEM micrographs of the PES and modified PES membranes. The characteristic morphology of the asymmetric membranes, consisting of a dense top layer and a porous bottom layer with a finger-like structure, was observed for all the modified membranes.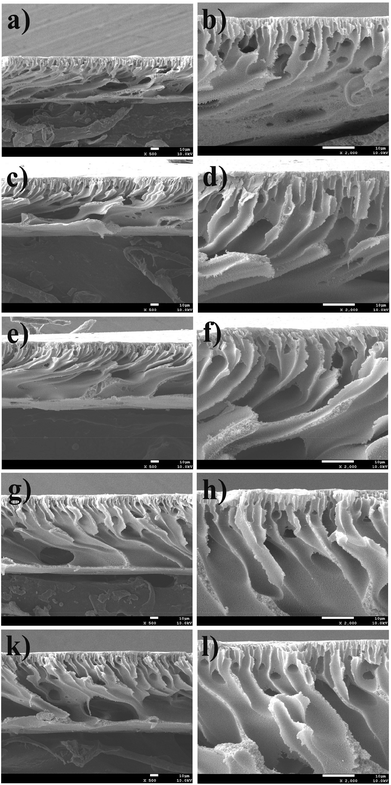 | ||
| Fig. 3 SEM images for the cross-section views of (a, b) M-2-1, (c, d) M-2-2, (e, f) M-2-3 and (g, h) M-2-4. The scale bars correspond to 10 μm in all the images. | ||
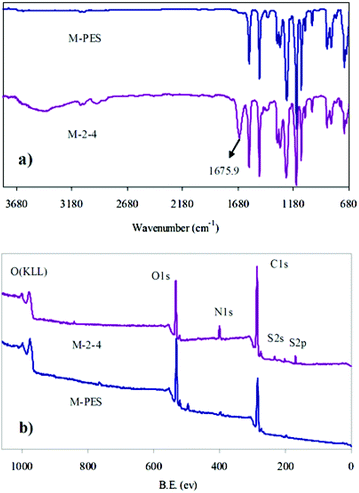
The surfaces of pure PES and the CLAC-2 modified PES membranes were investigated by ATR-FTIR and XPS, as shown in Fig. 4 and Table 3. It can be seen that the most significant changes of the CLAC modified PES membrane surface were: (i) the increased peak intensity at 1675.9 cm−1 (Fig. 4a), a characteristic peak of the CLAC; and (ii) the increased N element content (from 0 to 7.79%) (Fig. 4b and Table 3), a characteristic element of the CLAC. The surface FTIR and XPS analyses indicated that there was a high CLAC concentration, and a –PVP chain layer formed on the surface of the modified membrane.
| Sample | C (%) | O (%) | N (%) | S (%) | Others |
|---|---|---|---|---|---|
| M-PES | 73.49 | 23.13 | — | 3.27 | 0.11 |
| M-2-4 | 67.02 | 20.23 | 7.79 | 3.88 | 1.08 |
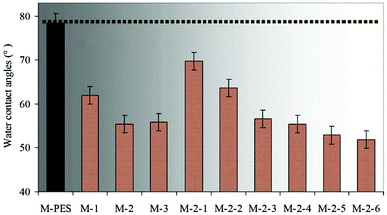
As is well known, the dynamic contact angle can better reflect the real surface wettability than the static contact angle (SCA). In this work, the hydrophilic PVP brushes formed on the membrane surface affected the measurement of the dynamic water contact angle. Thus, in order to simplify the experiment process, SCA was selected instead of the dynamic contact angle. The effects of the –PES chain Mn and the blended CLAC-2 amount on the surface composition and hydrophilicity of the modified membranes were investigated, as shown in Table 4 and Fig. 5. The XPS and SCA data showed that the elemental content of N increased and the SCA decreased with increasing the length of the –PES chain in the CLAC firstly. Then, the N content and the SCA for the modified membrane surfaces remained unchanged when the length of the –PES chain further increased. It is obviously seen that the copolymer of CLAC-2 with O-2 as a hydrophobic chain can effectively modify the PES membrane surface. With further increasing the length of the root-like chain, the effect was little. Thus, CLAC-2 was selected as the additive for PES modification, and modified membranes with 16 wt% PES and different CLAC-2 amounts were prepared. It is very interesting and surprising that the CLAC can be blended with PES at any ratio due to the good miscibility. As a result, all the casting solutions were uniform and transparent, and all the prepared membranes were smooth and regular. The XPS and SCA data indicated that the N content increased and the SCA decreased with the increase of the CLAC-2 amount in the blended membranes. The N content and SCA were invariable when the CLAC-2 amount was up to 8 wt%.
| Sample | M-PES | M-1 | M-2 | M-3 | M-2-1 | M-2-2 | M-2-3 | M-2-4 | M-2-5 | M-2-6 |
|---|---|---|---|---|---|---|---|---|---|---|
| CLAC content (wt%) | 0 | 33.33 | 33.33 | 33.33 | 5.89 | 15.79 | 23.81 | 33.33 | 42.86 | 50.00 |
| N (%) | — | 5.45 | 7.79 | 7.76 | 1.09 | 3.40 | 6.52 | 7.79 | 7.81 | 7.80 |
3.3 Hemocompatibility evaluation
Thrombus formation occurs soon after a material contacts blood, which will limit its application.22 It is well known that the hydrophobic interaction between a material surface and blood components plays an important role in thrombus formation.23 Materials possessing hydrophilic surfaces normally show relatively low adsorption and adhesion of proteins and cells. In addition, platelet adhesion and activation on the surfaces will trigger the coagulation of blood, leading to thrombus formation.24 A platelet adhesion experiment could reflect platelet adhesion and the deformation of the adhered platelets, while an APTT test could determine the bioactivity of intrinsic blood coagulation factors. Thus, platelet adhesion and the APTT test were used to investigate the hemocompatibility of the CLAC modified PES membranes.Fig. 6 shows the platelet adhesion on the membranes. It can be observed that numerous platelets aggregated and accumulated on the PES membrane surface; however, few platelets were observed on the M-2-4 surface. In order to verify the amount of adhering platelets on the membranes, platelet adhesion was also observed using the immunofluorescence method (see ESI†). The results indicated that the adhering platelets counted by SEM and by the immunofluorescence method were similar. In addition, the platelets adhering on the pure PES membrane had flattened and irregular shapes; however, the platelets adhering on the CLAC modified membrane had a rounded morphology with almost no pseudopodium. The results revealed that the platelets on the modified membrane were strongly suppressed and kept their original shapes, which should be attributed to the stable –PVP brush layer on the modified membrane surface.
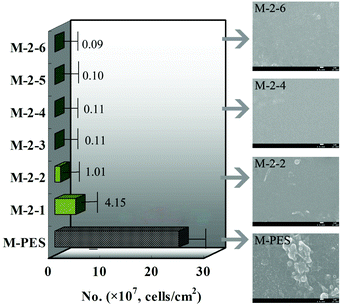 | ||
| Fig. 6 Numbers of the adherent platelets on the membranes adsorbed from platelet-rich plasma estimated from the SEM pictures. The scale bars correspond to 1 μm in all the images. | ||
Fig. 7 shows the APTT test results analyzed by statistical methods (significant difference, P < 0.05, compared with the APTT of the pristine PES membrane). It was found that the APTTs of the modified membranes were increased obviously compared with the pure PES membrane (P < 0.05). With increasing the CLAC-2 amount in the membrane, the APTT increased, being nearly doubled for the modified PES membrane with 5 wt% CLAC-2 amount. The enhancement in anticoagulant activity might have partially resulted from the surface hydrophilicity, low protein adsorption, and depressed platelet adhesion and activation, which were consistent with the results of the surface composition and wettability. However, when the CLAC-2 content further increased, the APTTs of the modified membranes stayed the same or slightly decreased. This might be caused by the equilibrium between the hydrophilicity and hydrophobicity. When the equilibrium was reached, the APTTs could not increase or decrease further. It should be pointed out that the outstanding performance was obtained for the modified PES membrane, which had been immersed in PBS at 37 °C for 30 days. To the best of our knowledge, this is the most effective blending method in improving anticoagulant activity and suppressing platelet adhesion, and it should be attributed to the improved surface hydrophilicity and low protein adsorption, due to the special structure of the copolymer.
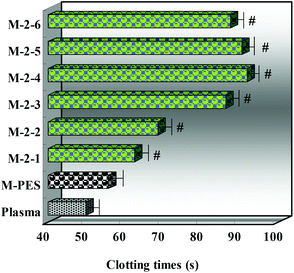 | ||
| Fig. 7 APTTs of the PES and modified membranes (n = 3; significant difference, P < 0.05, compared with the APTT of the pristine PES membrane). | ||
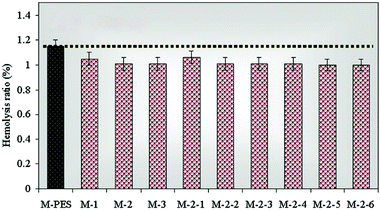
The hemolysis ratio is one of the key indicators to evaluate the blood compatibility of blood-contacting materials.25Fig. 8 shows the results of the hemolysis test. All the modified membranes presented low hemolysis ratios, ranging from 1.0 to 1.1%. It is well known that PVP has good blood compatibility. PVP is initially used as a blood plasma substitute and can later be applied in a wide variety of applications, such as biomaterials and coatings, disinfectants, intra-ocular lenses, medicine, pharmacy, cosmetics and industrial production.26 Thus, the modified membranes had anti-hemolytic activity due to the PVP brushes forming on the membrane surface.
3.4 Permeability to PEG solution
For end-stage renal disease patients, the removal of middle molecular weight toxins (such as β2-microglobulin) is necessary. β2-Microglobulin is considered as one of the middle weight toxins, and the molecular weight of β2-microglobulin is 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800. In this study, to investigate the removal of middle molecular weight toxin, PEG-10000 is selected in order to simulate the β2-microglobulin. The observed sieving coefficients of PEG-10000 for the PES and CLAC-2 modified PES membranes were 21.4% and 37.1%, respectively. These results suggested that the modified membrane could effectively remove the middle molecular weight toxin β2-microglobulin, and can be used as a high flux hemodialysis membrane for blood purification.
800. In this study, to investigate the removal of middle molecular weight toxin, PEG-10000 is selected in order to simulate the β2-microglobulin. The observed sieving coefficients of PEG-10000 for the PES and CLAC-2 modified PES membranes were 21.4% and 37.1%, respectively. These results suggested that the modified membrane could effectively remove the middle molecular weight toxin β2-microglobulin, and can be used as a high flux hemodialysis membrane for blood purification.
3.5 Self-assembly procedure and biofouling mechanism
The schematic representation of the membrane surface segregation layer architecture by assembly of the CLAC and the biofouling mechanism of the copolymer modified membranes can be briefly described as shown in Fig. 9. The initial solution, containing PES, CLAC and DMAC, formed a homogeneous solution; in this case, the CLAC macromolecules substantially dissolved in the solution. Because of the amphiphilic nature of the copolymer, when phase separation began, the CLAC moved to the surface of the membrane and self-assembled with the hydrophilic functional block directed to the surface and the hydrophobic part mingled in the membrane bulk.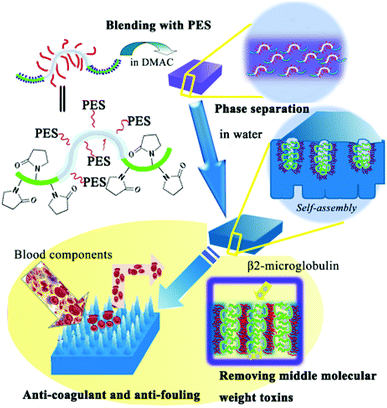 | ||
| Fig. 9 Schematic illustration for the preparation of a functional brush on the surface of the membrane and the interaction between blood constituents and the modified membrane surface. | ||
Hydrophilicity was the main driving force for chain migration and self-assembly of the copolymer during phase separation. However, it is very difficult to provide direct evidence for the data and analysis to support it. In fact, a series of amphiphilic triblock copolymers, such as PVP-b-PMMA-b-PVP, had been designed and synthesized to modify the PES membrane through chain migration and self-assembly using the phase separation technique.20 The surface compositions of the modified membranes were investigated using angle-resolved XPS, and the surface segregations of the membranes were compared and discussed by the van Oss–Chaudhury–Good approach to prove the statement indirectly. Phase separation occurred at the interface between PES and water in seconds due to the fast solvent exchange. The solvent exchange might be the underlying reason for the orientation of the amphiphilic copolymer with the hydrophilic chains being present at the surface and the hydrophobic chains being embedded in the substrate. It was found that during the phase separation, hydrophilicity was the main driving force for the migration and self-assembly of the block copolymer. In case of the synthesized amphiphilic copolymer CLACs in the present paper, hydrophilicity was also the greatest driving force for chain migration and self-assembly. The hydrophilic PVP chains were also present at the surface of the modified membranes, since phase separation occurred at the interface between PES and water. Furthermore, the surface energies of M-1, M-2 and M-3 measured using the van Oss–Chaudhury–Good method were 46.6, 47.0 and 47.4 mJ m−2, respectively, and the values were similar to that of PVP (48.8 mJ m−2). It could be concluded that a functional chain layer was formed on the surface of the modified membrane.
It is noteworthy that the surface includes the membrane surface and the membrane pore surface. When blood contacts the membrane during blood purification, all the events happen on the surface of the membrane. The obtained functional chain layer can prevent the adsorption and adhesion of blood components and endow the membrane with low protein adsorption, suppressed platelet adhesion, prolonged blood coagulation time and increased permeability. In view of the design ability of the CLAC, various kinds of functional surfaces, such as negative charged surface, heparin-like surface and pH sensitive surface, could be constructed by a simple blending method.
4. Conclusions
In conclusion, comb-like amphiphilic copolymers (CLACs) were synthesized, and novel and hydrophilic membranes were constructed. The prepared CLACs can be blended with PES at any ratio, and the prepared membranes were smooth and regular due to the good miscibility of the CLAC and PES. The CLAC modified membranes showed increased hydrophilicity, suppressed platelet adhesion, and prolonged blood clotting time. The results indicated that the modified membranes had improved blood compatibility and good permeability, and might have tremendous potential for blood purification.Acknowledgements
This work was financially sponsored by the National Natural Science Foundation of China (no. 51073105, 51173119 and 51225303) and State Education Ministry of China (Doctoral Program for High Education, no. 20100181110031). We should also thank our laboratory members for their generous help, and gratefully acknowledge the help of Ms H. Wang of the Analytical and Testing Center at Sichuan University for the SEM and Ms Liang of the Department of Nephrology at West China Hospital for the human fresh blood collection.Notes and references
- (a) S. R. Meyers and M. W. Grinstaff, Chem. Rev., 2012, 112, 1615–1632 CrossRef CAS PubMed; (b) S. Maddula and J. Ansell, J. Thromb. Thrombolysis, 2013, 36, 203–211 CrossRef CAS PubMed.
- (a) N. A. Peppas and R. Langer, Science, 1994, 263, 1715–1720 CAS; (b) G. Tripisciano, A. Leistner, L. Linsberger, A. Lerstner, D. Falkenhagen and V. Wever, Biomacromolecules, 2012, 13, 484–488 CrossRef PubMed.
- (a) R. S. Meyers and M. W. Grinstaff, Chem. Rev., 2012, 112, 1615–1632 CrossRef PubMed; (b) M. Tanaka, A. Mochizuki, N. Ishii, T. Motomura and T. Hatakeyama, Biomacromolecules, 2002, 3, 36–41 CrossRef CAS PubMed.
- B. M. Gorbet and V. Michael, Biomaterials, 2004, 25, 5681–5703 CrossRef PubMed.
- (a) Y. Zhuan, L. P. Zhu, L. Cheng, B. K. Zhu and Y. Y. Xu, Polymer, 2012, 53, 350–358 CrossRef PubMed; (b) X. L. Liu, Y. J. Xu, Z. Q. Wu and H. Chen, Macromol. Biosci., 2012, 13, 147–154 CrossRef PubMed; (c) M. Ulbricht, H. Matuschewski, A. Oechel and G. H. Hicke, J. Membr. Sci., 1996, 115, 31–47 CrossRef CAS; (d) L. P. Zhu, Z. Yi, F. Liu, X. Z. Wei, B. K. Zhu and Y. Y. Xu, Eur. Polym. J., 2008, 44, 1907–1914 CrossRef CAS PubMed.
- H. E. Achneck, R. M. Jamiolkowski, A. E. Jantzen, J. M. Haseltine, W. O. Lane, J. K. Huang, L. J. Galinat, M. J. Serpe, F. H. Lin, M. Li, A. Parikh, L. Ma, T. Chen, B. Sileshi, C. A. Milano, C. S. Wallace, T. V. Stabler, J. D. Allen, G. A. Truskey and J. H. Lawson, Biomaterials, 2011, 32, 10–18 CrossRef CAS PubMed.
- W. Khan, M. Kapoor and N. Kumar, Acta Biomater., 2007, 3, 541–549 CrossRef CAS PubMed.
- (a) S. Q. Nie, M. Tang, C. Cheng, Z. H. Yin, L. R. Wang, S. D. Sun and C. S. Zhao, Biomater. Sci., 2014, 2, 98–109 RSC; (b) X. J. Huang, D. Guduru, Z. K. Xu, J. Vienken and T. Groth, Macromol. Biosci., 2011, 11, 131–140 CrossRef CAS PubMed.
- S. H. Ye, J. J. Watanabea, M. Takaia, Y. Iwasakib and K. Ishihara, Biomaterials, 2005, 26, 5032–5041 CrossRef CAS PubMed.
- (a) Q. Lv, C. B. Cao and H. Zhu, Polym. Int., 2005, 54, 1076–1081 CrossRef CAS; (b) P. Klement, Y. J. Du, L. Berry, M. Andrew and A. K. C. Chan, Biomaterials, 2002, 23, 527–535 CrossRef CAS; (c) Q. Wei, F. L. Zhang, J. Li, B. J. Li and C. S. Zhao, Polym. Chem., 2010, 1, 1430–1433 RSC; (d) C. S. Zhao, J. M. Xue, F. Ran and S. D. Sun, Prog. Mater. Sci., 2013, 58, 76–150 CrossRef CAS PubMed; (e) Y. S. Chi, B. S. Lee, M. Kil, H. J. Jung, E. Oh and I. S. Choi, Chem. Asian J., 2009, 4, 135–142 CrossRef CAS PubMed; (f) P. S. Liu, Q. Chen, X. Liu, B. Yuan, S. S. Wu, J. Shen and S. C. Lin, Biomacomolecules, 2009, 10, 2809–2816 CAS.
- J. Yuan, J. Zhang, X. P. Zang, J. Shen and S. Lin, Colloids Surf., B, 2003, 30, 147–155 CrossRef CAS.
- (a) A. Higuchi, K. Shirano, M. Harashima, B. O. Yoon, M. Hara, M. Hattori and K. Imamura, Biomaterials, 2002, 23, 2659–2666 CrossRef CAS; (b) S. Q. Nie, J. M. Xue, Y. Lu, Y. Q. Liu, D. S. Wang, S. D. Sun, F. Ran and C. S. Zhao, Colloids Surf., B, 2012, 100, 116–125 CrossRef CAS PubMed; (c) S. H. Ye, J. Watanabe, M. Takai, Y. Iwasaki and K. Ishihara, Biomaterials, 2006, 27, 1955–1962 CrossRef CAS PubMed.
- (a) Y. Tamada, M. Murata, K. Goto and T. Hayashi, Biomaterials, 2002, 23, 1375–1382 CrossRef CAS; (b) F. Ran, S. Q. Nie, Z. H. Yin, J. Li, B. H. Su, S. D. Sun and C. S. Zhao, Int. J. Biol. Macromol., 2013, 55, 269–275 CrossRef CAS PubMed.
- (a) D. K. Han, N. Y. Lee, K. D. Park, Y. H. Kim, H. I. Cho and B. G. Min, Biomaterials, 1995, 16, 467–471 CrossRef CAS; (b) F. Ran, S. Q. Nie, J. Li, B. H. Su, S. D. Sun and C. S. Zhao, Macromol. Biosci., 2012, 12, 116–125 CrossRef CAS PubMed.
- L. S. Wan, Z. K. Xu and Z. G. Wang, J. Polym. Sci., Part B: Polym. Phys., 2006, 44, 1490–1498 CrossRef CAS.
- S. Krishnan, R. Ayothi, A. Hexemer, J. A. Finlay, K. E. Sohn, R. Perry, C. K. Ober, E. J. Kramer, M. E. Callow, J. A. Callow and D. A. Fischer, Langmuir, 2006, 22, 5075–5086 CrossRef CAS PubMed.
- T. L. John, F. Debby and S. Ronald, Macromolecules, 2002, 35, 6754–6756 CrossRef.
- B. Qian, J. Li, Q. Wei, P. L. Bai, B. H. Fang and C. S. Zhao, J. Membr. Sci., 2009, 344, 297–303 CrossRef CAS PubMed.
- X. W. Gong, D. Z. Wei, M. L. He and Y. C. Xiong, Talanta, 2007, 71, 381–384 CrossRef CAS PubMed.
- T. Miyano, T. Matsuura and S. Sourirajan, Chem. Eng. Commun., 1993, 119, 23–39 CrossRef CAS.
- F. Ran, S. Q. Nie, W. F. Zhao, J. Li, B. H. Su, S. D. Sun and C. S. Zhao, Acta Biomater., 2011, 7, 3370–3381 CrossRef CAS PubMed.
- B. Yameen and A. Farrukh, Chem. Asian J., 2013, 8, 1736–1753 CrossRef CAS PubMed.
- Y. P. Jane, H. A. Metin, A. Ariya, W. Kuhlman and A. M. Mayes, Biomaterials, 2006, 27, 856–865 CrossRef PubMed.
- N. Mackman, Nature, 2008, 451, 914–918 CrossRef CAS PubMed.
- J. Li, S. Q. Nie, L. R. Wang, S. D. Sun, F. Ran and C. S. Zhao, J. Appl. Polym. Sci., 2013, 130, 4284–4298 CAS.
- (a) F. Haaf, A. Sanner and F. Straub, Polym. J., 1985, 17, 143–152 CrossRef CAS; (b) K. A. George, E. Wentrup-Byrne, D. J. T. Hill and A. K. Whittaker, Biomacromolecules, 2004, 5, 1194–1199 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3bm60250h |
| This journal is © The Royal Society of Chemistry 2014 |