Jigsaw cooperative learning: Acid–base theories
Leman
Tarhan
*a and
Burcin
Acar Sesen
b
aScience Faculty, Chemistry Department, Dokuz Eylul University, 35160 Buca, Izmir, Turkey. E-mail: leman.tarhan@deu.edu.tr
bHasan Ali Yucel Education Faculty, Department of Science Education, Istanbul University, 34452 Eminonu, Istanbul, Turkey. E-mail: bsesen@istanbul.edu.tr
First published on 16th April 2012
Abstract
This study focused on investigating the effectiveness of jigsaw cooperative learning instruction on first-year undergraduates' understanding of acid–base theories.Undergraduates' opinions about jigsaw cooperative learning instruction were also investigated. The participants of this study were 38 first-year undergraduates in chemistry education department in an education faculty in Izmir, Turkey. A prerequisite knowledge test was applied to both experimental (N = 18) and control groups (N = 20) before the treatment in order to identify undergraduates' prerequisite knowledge about ‘acids and bases’. Independent t-test was conducted to compare the prerequisite knowledge test scores for groups and no significant difference was found in terms of mean scores (t = 0.42, p > 0.05). The subject of “Acid–Base Theories” (Arrhenius, Brønsted–Lowry and Lewis Theories) was taught using jigsaw cooperative learning in the experimental group and with regular teacher-centered approach in the control group. After the instruction, the acid–base theories concept test was administrated to investigate undergraduates' conceptual understanding. Independent t-test results showed significant difference in terms of mean scores (t = 4.65, p < 0.05).The results also indicated that undergraduates in the experimental group had fewer misconceptions and understood the concepts more meaningfully than undergraduates in the control group. In addition, individual interviews reflected that undergraduates had positive opinion about jigsaw, and they believed jigsaw is an effective cooperative learning technique that promotes positive attitudes and interest, develop inter personal skills as well as their learning achievements.
Introduction
It is well known that students can develop their own ideas about scientific phenomenon based on many factors such as real-world experiences, media, books and interaction with people (Driver and Erickson, 1983). According to constructivism, learning occurs in the mind of learner, and during this process students integrate their prior knowledge with new knowledge (Bodner, 1986). If their existing conceptions are different from the scientific view, misconceptions are formed (Osborne and Freyberg, 1985; Nakhleh, 1992). From this view, students' misconceptions can interfere with students' learning of correct scientific principles or concepts (Driver and Erickson, 1983; Taber, 2000). Researchers have asserted that students in the teacher-centered classes could not achieve the adequate conceptual understanding (Acar Sesen and Tarhan, 2011; Hsu, 2008; Kaya, 2007). Therefore, they claimed that students should be engaged active learning environments (Acar and Tarhan, 2007, 2008; Doymus, 2008a, b; Hand and Treagust, 1991; Sisovic and Bojovic, 2000). Cooperative learning is one of these active learning approaches in which students in the small groups work together to complete an assigned task (Cooper and Mueck, 1990).Researchers have indicated benefits of cooperative learning as higher academic achievement, greater persistence through graduation, higher level reasoning and critical thinking skills, deeper understanding of learned material, better attention and less disruptive behavior in class, lower amounts of anxiety and stress, more motivation to learn and achieve, positive attitudes to subject matter, higher self esteem (Cooper and Mueck, 1990; Johnson et al., 1991; McKeachie, 1986). This shows that students achieve more, improve their social skills, and increase their capacity to work productively together while working in cooperative learning environment.
There are many cooperative learning techniques and most widely used of them are Student Teams-Achievement Division (STAD; Slavin, 1980), Teams-Games-Tournament (TGT; Slavin, 1980), Learning Together (Johnson and Johnson, 1994); and Group Investigation (Sharan and Hertz-Lazarowitz, 1980), Jigsaw (Aronson et al., 1978.; Slavin, 1980). The cooperative learning technique selected for this study is jigsaw, which enhances cooperative learning by making each student responsible for teaching some of the learning issues to the group. In this structure, students are members of two different groups, the ‘home group’ and the ‘jigsaw group’ (Fig. 1). Initially, students meet in their home groups and each member of the group is assigned a portion of the learning issues to learn as an ‘expert’ (Slavin, 1980). The home groups then break apart, like pieces of a jigsaw puzzle, and students move into jigsaw groups, which consist of members from the other home groups who have been assigned the same portion of the learning issues. While in the jigsaw groups, the students discuss their particular material to ensure that they understand it. Students then return to their home groups, where they teach their material to the rest of their group (Colosi and Zales, 1998).
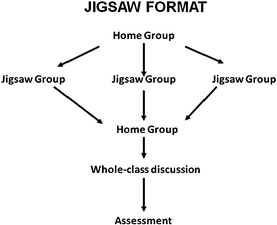 | ||
| Fig. 1 The jigsaw cooperative learning process. | ||
Researchers underlined that jigsaw is an effective cooperative learning technique that promotes positive attitudes and interests in the learning issues, development of communication skills between students and also higher learning achievement in science (Lazarowitz et al., 1985; Colosi and Zales, 1998; Doymus, 2008a; Eilks, 2005; Young et al., 1997). In the field of chemistry education, relatively little research has been done on the use of jigsaw techniques. In the one of the study, Eilks (2005) discussed using a modified jigsaw-classroom method to teach atomic structure in the 9th and 10th grades, and found that the jigsaw techniques have potential to improve students' attitude towards science. Doymus (2008a) investigated the effect of jigsaw cooperative learning versus individual learning methods on students' understanding of chemical equilibrium in a first-year general chemistry course and found that students in the jigsaw class were more successful than those in the individual learning class. In another study, Doymus (2008b) examined the effectiveness of jigsaw technique to teach ionic bonding, covalent bonding, hydrogen bonding and van der Waals forces, and found the same results as his previous study.
The subject of acids and bases are also an important and fundamental concept in chemistry learning as early as primary school, right through the university level. Researchers have shown that students have many misconceptions related to acid and base theories (Banerjee, 1991; Bradley and Mosimege, 1998; Cross et al., 1986; Rayner-Canham, 1994; Schmidt, 1995).Bradley and Mosimege (1998) asserted that university students have problems with Arrhenius theory. They found that 38% of them were able to identify the important objection against the theory. Cross et al., (1986) also indicated that 47% of the first-year university students gave the Brønsted–Lowry definition and only 14% gave the Arrhenius definitions of acids. Vidyapati and Seetharamappa (1995) investigated higher secondary school students' concepts of acids and bases. In this study, it was found that the percentage of students citing the right examples of acids and bases according to the Arrhenius, Brønsted–Lowry and Lewis theories is more than the percentage of students who gave the scientific definitions, and they suggested cooperative learning in place of normal lecture classes to overcome this ineffectively construction of knowledge. Hawkes (1992) observed that the Arrhenius acid–base theory confused students, and when asked to use the Brønsted theory, which applies to a variety of bases, students' thinking was still dominated by the Arrhenius theory, in which only OH− ion-producing substances are considered as bases. For this reason Hawkes (1992) suggested that the Brønsted theory should be introduced first, and that the Arrhenius theory should only be used as a historical footnote. In the other hand, Demerouti et al., (2004) reported that students from upper secondary school were more familiar with the Arrhenius theory, and they did not use the Brønsted theory to explain the properties of acids and bases.
As a conclusion, the results of those studies underlined that students have some difficulties and misconceptions about acid and base theories.
Purpose of the research
The purpose of this study was to investigate the effectiveness of jigsaw cooperative learning instruction on first-year undergraduates' understanding of ‘acid and base theories'. Undergraduates' opinions about jigsaw cooperative learning were also investigated in the context of this study. For this reason, the following research questions were investigated;What is the first-year undergraduates' prerequisite knowledge about acid and base theories at the beginning of the instruction?
Does jigsaw cooperative learning contribute to better conceptual understanding of acid and base theories in first-year undergraduates' than teacher-centered approach?
What are the opinions of experimental group undergraduates about the treatment based on jigsaw cooperative learning?
Methodology
Participants
The participants of this study consisted of 38 first-year undergraduates (18–19 years of age) enrolled in a General Chemistry Course at chemistry teaching department in a faculty of education cited in Izmir, which is located in the west of Turkey. The undergraduates were from different cities in Turkey. The socio-economic status of the them was similar and the majority of them were from middle to upper class families. The undergraduates enroled in faculty of education to be a chemistry teacher based on the scores taken in university entrance examination.The place of acid and base theories in the turkish curriculum
The concepts of acids and bases are taught initially in the eighth grade (age 13–14) Science and Technology Lesson according to Turkish Educational System. Students learn the subject of acid and base theories in high school chemistry lesson. In this grade, the Arrhenius Theory is presented first, then Brønsted–Lowry Theory and finally, though not in as much detail, the Lewis Theory are explained. In the university level, the three theories are thought in a detailed in general chemistry lesson.Instruments
Acids and bases are related to many other chemistry concepts such as atom, molecule, solubility, solution, the periodic table, electronegativity, chemical bonding, chemical reactions, thermodynamics, and chemical equilibrium. Researchers have underlined that the causes of student difficulties in acid–base chemistry have been ascribed to the existence of many misconceptions related to these aforementioned concepts (Demircioğlu, 2003; Nakhleh and Krajcik, 1993; Nakhleh, 1994; Smith and Metz, 1996; Schmidt, 1997; Sheppard, 1997). For this reason, in this study, the prerequisite knowledge test consisting of 16 multiple-choice items was developed by the researchers to identify undergraduates' understanding the concepts that effects learning of acids and bases. The items of the test were constructed by considering students' misconceptions determined in the literature (Ebenezer and Gaskell, 1995; Griffiths and Preston, 1992; Peterson et al., 1989; Sanger, 2000). Each item had one correct answer and four incorrect answers (distracters). The distracters are derived from actual student misconceptions gathered from the literature. For the content validity and error reduction, the items were evaluated by seven chemistry educators. The prerequisite knowledge test was piloted with the sample of 152 undergraduates for the reliability. After the item analysis the reliability coefficient (KR-20) of the test was found to be 0.77.
Each answer was evaluated by researchers and two expert chemistry instructors. The scores were compared and discussed until an agreement was reached.
What is your opinion about the effects of jigsaw cooperative learning application on you and your friends' chemistry achievements?
If you compare the jigsaw cooperative learning technique to the teacher centered approach, how can you explain the differences about the learning process and the roles of instructor's and students?
Procedure
38 undergraduates were randomly assigned to experimental (N = 18) and control groups (N = 20). Before the instruction, the prerequisite knowledge test was applied to both groups to identify undergraduates' pre-knowledge related to the ‘acid and base theories'. The results revealed that there was no significant difference between mean scores of groups (Table 1). The experimental group was taught using jigsaw cooperative learning and control group was taught using traditional course content based on teacher-centred instruction during the same instructional period. These two groups were instructed by the same competent chemistry instructor.Instruction in the experimental group
Before the instruction, undergraduates in the experimental group were informed about cooperative learning and jigsaw technique. Their and instructors' responsibilities, utilization of resources were also explained.In an cooperative learning environment, group formation is very important for success. In this study, stratified random sampling was used as a method of group formation. This method of group formation involves creating small subgroups (strata) of undergraduates stratified along a specific dimension and then randomly choosing group members from each of these strata. By design, stratified random sampling yields groups that are balanced across the dimension used to form the strata (Fraenkel and Wallen, 2005). In this study, the undergraduates in the experimental group, were stratified random to six home groups considering their chemistry achievements. As presented in Fig. 2, there were three undergraduates in each home group. Each member of the groups was assigned a portion of acid–base theories and then they moved into three jigsaw groups including six members to be expert. Jigsaw group-1 studied Arrhenius Acid–Base Theory, jigsaw group-2 investigated Brønsted–Lowry Acid–Base Theory, and jigsaw group-3 searched Lewis Acid–Base Theory. During this process, they were encouraged to develop their hypothesis and to make task distributions in the fifteen minute period under the guidance of the instructor. Then, they were directed to study their own subtopics outside the class. Undergraduates benefited from library, textbooks and internet, and they worked under the supervision of the instructor to achieve the learning objectives.Undergraduates then returned their home groups and taught their own expertise subtopics to the rest of their group.
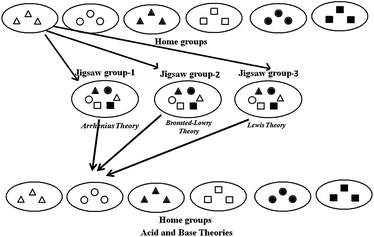 | ||
| Fig. 2 Studies in home and jigsaw groups. | ||
Jigsaw group-1. First jigsaw group was responsible to study Arrhenius Acid and Base Theory. After their research about the subtopic outside the class, they discussed their findings and made a presentation in their group under the guidance of the instructor. In this way, undergraduates would able to explain;
• Arrhenius investigation on galvanic conductivity of electrolytes,
• salts dissociate when they dissolve in water to give charged particles which Arrhenius called ions,
• why acids have similar characteristics, since they all give H+ ions when they dissolve in water,
• why bases have similar characteristics, since they all give OH− ions when they dissolve in water,
• ionization equation of Arrhenius acid and bases in the water,
• identification of Arrhenius acid and bases,
• the limits of Arrhenius theory.
Jigsaw group-2. The second jigsaw group studied Brønsted–Lowry Acid and Base Theory. In the second lesson, the group members discussed their studies in their group under the guidance of the instructor. After this study that all the undergraduates would able to explain;
• Brønsted and Lowry separately proposed a new set of definitions for acids and bases,
• acids are any substance that can donate H+ ion to a base,
• bases are any substance that can accept H+ ion from an acid,
• the role of water in acid–base reactions,
• the reason of formation hydronium ion in the water,
• dissociation of Brønsted and Lowry acid and bases in the water,
• part of the acid remaining when an acid donates a H+ ion is called the conjugate base and the acid formed when a base accepts a H+ ion is called the conjugate acid,
• acids and bases can be ions or neutral molecules according to Brønsted and Lowry theory,
• acid and bases can be applied to solutions with solvents other than water and even in reactions that occur in the gas or solid phases,
• the limitations of Brønsted–Lowry theory.
Jigsaw group-3. The third jigsaw group was studied on Lewis Acid and Base Theory. After their study outside the class, they presented their studies in their group under the guidance of the instructor. In this way, undergraduates would able to explain;
• Lewis acids are any substance that can accept a pair of nonbonding electrons,
• Lewis bases are any substance that can donate a pair of nonbonding electrons,
• Lewis acids are those which can form a new covalent bond by accepting a pair of electrons and Lewis Bases are those that can form a new covalent bond by donating a pair of electrons,
• formation of complex ions according to Lewis theory,
• identification organic molecules that act as a Lewis acid or Lewis base,
• the limits of Lewis theory.
After the presentations of each jigsaw group, a 15 min break was taken. In the third lesson, undergraduates were moved to their home groups to complete their task related to the ‘Acid–Bases Theories’.
• Arrhenius theory can only classify substances when they are dissolved in water since the definitions are based upon the dissociation of compounds in water,
• Arrhenius theory does not explain why some compounds containing hydrogen such as HCl dissolve in water to give acidic solutions and why others such as CH4 do not,
• Arrhenius theory can only classify substances as bases if they contain the OH− ion and cannot explain why some compounds that don't contain the OH− such as Na2CO3 have base-like characteristics,
• Brønsted theory explains acids and bases can be ions or neutral molecules,
• Brønsted theory explains bases can be any molecule with at least one pair of nonbonding electrons,
• Brønsted theory explains the role of water in acid–base reactions; H2O accepts H+ ions from acids to form H3O+ ions,
• Brønsted theory can be applied to solutions with solvents other than water and even in reactions that occur in the gas or solid phases,
• Brønsted theory relates acids and bases to each other with conjugate acid–base pairs and can explain their relative strengths,
• Brønsted theory explains the relative strengths of pairs of acids or pairs of bases,
• Only Lewis theory explains formation of complex ions,
• Only Lewis theory explains organic molecules acid or base characterizes.
Instruction in the control group
Undergraduates in the control group were instructed via teacher-centered approach. This instruction included lecture, discussion and problem solving. Throughout the lesson in the control group, the same instructor presented the same content as the experimental group to achieve the same learning objectives that detailed instruction in the experimental group section. During this process instructor used blackboard and asked some questions related to the subject. Undergraduates also used regular textbook. While the instructor explained the subject, the undergraduates listened to her and took notes.They solved the problems related to Acid–Base Theories. In addition, the some problems were assigned as homework in order to ensure time equation in the experimental group.Results and discussion
As indicated before, researchers have asserted the reason of students' misconceptions in acids and bases are related their prior knowledge and learning difficulties about some chemistry subjects such as particulate nature of matter, solubility, chemical reactions, stoichiometry, ionic dissociation of substances, chemical bonding and chemical equilibrium (Furio-Más et al., 2007; Nakhleh and Krajcik, 1993; Nakhleh, 1994; Ross and Munby, 1991; Sheppard, 2006; Smith and Metz, 1996). For this reason, undergraduates' prior knowledge and possible misconceptions were identified via a prerequisite knowledge test. The independent sample t-test was used to compare the mean scores. As shown in Table 1, the mean scores of the experimental and control groups were 21 and 22 respectively, and there were no significant differences in terms of prerequisite knowledge test (t = 0.4, p > 0.05).Undergraduates' responses to the prerequisite knowledge test indicated that they had misconceptions related to identification chemical bonds and inter molecular forces, confusion ionic and covalent bonds, confusion London and dipol-dipol forces, solubility, and chemical equilibrium (Table 2). Because these concepts were important for learning ‘Acid and Base Theories’, undergraduates in both group were taught these concept before and during the instruction.
| Group | N | Mean | SD | t | p |
|---|---|---|---|---|---|
| Experimental | 18 | 21 | 7.4 | 0.4 | 0.68 |
| Control | 20 | 22 | 5.4 |
| Undergraduates' misconceptions | Exp. Grp (%) | Cont. Grp (%) |
|---|---|---|
| HCl is an ionic compound. | 67 | 70 |
| H2 includes hydrogen bonds. | 44 | 30 |
| London forces are stronger than dipole–dipole forces. | 56 | 40 |
| Because HF molecule can ionize in the water, it has high solubility. | 67 | 75 |
| If equilibrium constant bigger than 1, the reaction occurs more rapidly. | 33 | 40 |
| All the acids and bases are strong electrolyte. | 50 | 60 |
| Hydrogen is a metal in I-A group of the periodic table. | 55 | 55 |
| Only solutions of ionic compounds conduct electricity. | 39 | 40 |
Instruction of Arrhenius, Brønsted–Lowry, and Lewis Acid–Base Theories was conducted with jigsaw cooperative learning in the experimental group and with teacher-centered approach in the control group. Immediately after the instructions the acid–base theories concept test was administrated to determine undergraduates' understanding. The independent sample t-test results showed that the undergraduates who trained with jigsaw cooperative learning significantly had higher scores than those taught by teacher-centred approach in terms of acid–base theories concept test mean scores (t = 4.6, p < 0.05, Table 3).
| Group | N | Mean | SD | t | p |
|---|---|---|---|---|---|
| Experimental | 18 | 24 | 4.1 | 4.6 | 0.002 |
| Control | 20 | 16 | 6.7 |
Undergraduates' responses to each item in the acid–base theories concept test reflected that undergraduates in the experimental group had significantly fewer misconception and understood ‘Acids and Bases Theories’ more meaningfully than undergraduates in the control group (Table 4). While two of the misconceptions in Table 4 were first identified in this study, six of them had been previously documented in the literature.
| Undergraduates' misconceptions | Exp. Grp(%) | Cont. Grp(%) |
|---|---|---|
| a Firstly identified misconceptions in this study. | ||
| Because HS− has hydrogen, it is Lewis acid. | 0 | 45 |
| Because HS− give its proton, it is Brønsted–Lowry acids. | 6 | 40 |
| CN− ion takes proton from the base and thereby it is Arrhenius base. | 0 | 35 |
| There is no electron transfer between NH3 and BF3 molecules. | 6 | 40 |
| Acids are the substances that only give H+ ions and bases are the substances that only gave OH− ions. | 11 | 45 |
| Bases are the substances that give proton and acids are the substances that gain proton. | 11 | 50 |
| Arrhenius theory explains transferring of H+ and Brønsted–Lowry theory explains transferring proton.a | 6 | 55 |
| According to Lewis Theory, ions should be combined to make new products.a | 0 | 45 |
Results reflected that undergraduates in the control group commonly had difficulties about Lewis Theory, and they confused Arrhenius and Brønsted–Lowry Theories. For example, one of the item, it is asked to undergraduates to identify the acids and bases in the reaction of HS− + CH3Cl → CH3SH + Cl− according to ‘Acid–Base Theories’. It was required undergraduates to answer this item as HS− is Lewis base, because it is an electron pair donor. While undergraduates in the experimental group correctly answered this item, 85% of the undergraduates in the control group gave the wrong answer. 45% of them identified HS− as Lewis acid because of having hydrogen. 40% of them thought that HS− is Brønsted–Lowry acids, because of giving proton.
In the other item, undergraduates were required to explain some sample reactions according to Lewis Theory. 45% of the undergraduates in the control group classified the reaction between NH3 and BF3 molecules as Lewis Theory, because they believed that new complex products as BF3NH3 should be formed according to Lewis theory. Those undergraduates also could not explain the reaction between CN− and H2O molecules deped on Lewis Theory. In the other hand, 40% of undergraduates in the control group could not explain this reaction according to Lewis theory and they indicated that there is no electron transfer between NH3 and BF3 molecules.
Those misconceptions underlined that over 35% of the undergraduates in the control group did not understand electron transfer between acids and bases depend on Lewis theory, could not explain the basic characteristics of some samples do not include OH− ions, confused H+ ion and proton. This situation indicate that undergraduates commonly prefer to define acid and base according to Brønsted–Lowry Theory, and had difficulties in explaining Lewis acid–base theories and confused acid and base theories with each other as mentioned by the other researches (Bradley and Mosimege, 1998; Demerouti et al., 2004; Zoller, 1990). This can be caused because the regular chemistry curriculum generally highlights Arrhenius and Brønsted–Lowry Theory, and the differences between these theories do not give apprehensible as indicated in the previous studies by Carr (1984), Schmidt (1995), Vidyapati and Seetharamappa (1995).
In the light of the results of this study, it can be said that jigsaw cooperative learning instruction is successful in improving students' conceptual understandings and preventing misconceptions. The findings are consistent with earlier studies as those of Doymus (2008a, 2008b), Eilks (2005) which revealed that the jigsaw method leads to higher achievement.
In order to identify undergraduates' opinions about jigsaw cooperative learning application, 15-minute period semi-structured individual interviews were conducted with all the undergraduates in the experimental group after the instruction. As shown in Table 5, undergraduates indicated that this instruction positively effected their attitudes towards chemistry, learning achievements, responsibilities, and social skills. 67% of the undergraduates indicated that jigsaw cooperative learning increased their chemistry achievements, and 61% of them began to think that chemistry is not memorization. In the other hand only 44% of them required using jigsaw cooperative learning in all the lessons. This result underlined that although undergraduates recognize the power of jigsaw cooperative learning, more than half of them do not want to be taught via jigsaw. They also do not want to study in group. This results underlined that undergratuates are not accustomed to this type pf learning. Threfore, application of jigsaw or the other cooperative learning techniques should be used most widely in chemistry and science classes. Additionally, undergarduates generally have positive attitudes and interest towards jigsaw techniqe. These findings are in agreement with previous research findings which revealed that the jigsaw method increased students' attitudes and interest (Dori 1995; Doymus et al., 2004).
| Experimental Group Undergraduates' Opinions about Jigsaw | (%) |
|---|---|
| Using jigsaw cooperative learning in all the chemistry lessons may increase my chemistry achievement. | 67 |
| Because we shared our ideas and knowledge, I learned better. | 50 |
| I learned chemistry is not memorization. | 61 |
| I wish jigsaw cooperative learning is used in all the lessons. | 44 |
| I learned the research techniques in the library and internet. | 72 |
| I began to like chemistry after jigsaw cooperative learning instruction. | 56 |
| Working with friends increased my interest to chemistry. | 44 |
| Instructor's monitoring helped us to plan our research. | 78 |
| I would rather educated by instructor than studying in the group. | 22 |
| The feedback given by the instructor helped us to reduce errors in our study. | 83 |
| I feel my confidence level in investigating has improved after the group study. | 61 |
| I enjoyed while working in my group. | 78 |
| I liked to study with my friends in the groups. | 44 |
| Working in groups developed the relations between friends. | 50 |
Conclusions
The present study was conducted to investigate the effectiveness of jigsaw cooperative learning instruction over teacher-centered approach on first-year undergraduates' understanding of ‘Acid and Base Theories’. The results reflected that jigsaw is an effective cooperative learning technique that promotes positive attitudes and interest, develop inter personal skills, and increase conceptual understanding. Additionally, there are limited studies on students' understanding of chemistry concepts via jigsaw cooperative learning. Therefore, it is believed that this study will contribute to the chemistry education literature. In the light of the results, it is suggested that jigsaw cooperative learning should be used widely in chemistry instruction.References
- Acar Sesen B. and Tarhan L., (2011), Active-learning versus teacher-centered instruction for learning acids and bases, Research in Science & Technological Education, 29, 205–226.
- Acar B. and Tarhan L., (2007), Effect of cooperative learning strategies on students' understanding of concepts in electrochemistry, International Journal of Science and Mathematics Education, 5, 349–373.
- Acar B. and Tarhan L., (2008). Effects of cooperative learning on students' understanding of metallic bonding, Research in Science Education, 38, 401–420.
- Aronson E., Blaney N., Stephan C., Sikes J. and Snapp M., (1978), The Jigsaw Classroom, Beverly Hills, CA, Sage Pub.
- Banerjee A. C., (1991), Misconception of students and teachers in chemical equilibrium, International Journal of Science Education, 13, 487–494.
- Bodner G., (1986), Constructivism: A theory of knowledge, Journal of Chemical Education, 63, 873–878.
- Bradley J. D. and Mosimege M. D., (1998), Misconceptions in acids and bases: a comparative study of student teachers with different chemistry backgrounds, South African Journal of Chemistry, 51, 137–145.
- Carr M., (1984), Model confusion in chemistry, Research in Science Education, 14, 97–103.
- Colosi J. C. and Zales C.R., (1998), Jigsaw Cooperative Learning Improves Biology Lab Course, Bioscience, 48, 118–124.
- Cooper J. L. and Mueck R., (1990), Student involvement in learning Cooperative learning and college instruction, Journal on Excellence College Teaching, 1, 68–76.
- Cross D., Maurin M., Amouroux R., Chastrette M., Leber J. and Fayol M., (1986), Conceptions of first-year university students' of the constituents of matter and the notions of acids and bases, European Journal of Science Education, 8, 305–313.
- Demerouti M., Kousathana M. and Tsaparlis G., (2004), Acid–base equilibria, part I. upper secondary students' misconceptions and difficulties, Chemical Educator, 9, 122–131.
- Demircioğlu G., (2003), Lise II Asitler ve bazlar nitesi ile ilgili rehber materyal gelitirilmesi ve uygulanması (Doctoral dissertation, Karadeniz Teknik University, Turkey). Retrieved from http://www.yok.gov.tr/content/view/59/111/lang,tr/.
- Dori Y. J., (1995), Cooperative development of organic chemistry computer assisted instruction by experts, teachers and students, Journal of Science Education and Technology, 4, 163–170.
- Doymus K., (2008a), Teaching chemical equilibrium with the jigsaw technique, Research in Science Education, 38, 49–260.
- Doymus K., (2008b), Teaching chemical bonding through jigsaw cooperative learning, Research in Science & Technological Education, 26, 47–57.
- Doymus K., Simsek U. and Bayrakceken S., (2004), The effect of cooperative learning on attitude and academic achievement in science lessons, Journal of Turkish Science Education, 2, 103–113.
- Driscoll M. P., (2005), Psychology of learning for instruction (3rd edn). Boston: Pearson.
- Driver R. and Bell B., (1986), Students' thinking and the learning of science: A constructivist view, School Science Review, 67, 443–456.
- Driver R. and Erickson G., (1983), Theories-in-Action: Some theoritical and emprical isues in the study of students' conceptual framework in science, Studies in Science Education, 10, 37–60.
- Ebenezer J.V. and Gaskell P. J., (1995), Relational conceptual change in solution chemistry, Science Education, 79, 1–17.
- Eilks I., (2005), Experiences and reflections about teaching atomic structure in a jigsaw classroom in lower secondary school chemistry lessons, Journal of Chemical Education, 82, 313–9.
- Fraenkel J. R. and Wallen N. E., (2005), How to Design and Evaluate Research in Education, New York: McGraw-Hill.
- Furio-Más C., Calatayud M. L. and Bárcenas S. L., (2007), Surveying students' conceptual and procedural knowledge of acid–base behavior of substances, Journal of Chemical Education, 84, 1717–1724.
- Griffiths A. K. and Preston K. R., (1992), Grade 12 students' misconceptions relating to fundamental characteristics of atoms and molecules, Journal of Research in Science Teaching, 29, 611–628.
- Hand B. and Treagust D. F., (1991), Student achievement and science curriculum development using a constructive framework, School Science and Mathematics, 91, 172–76.
- Hsu Y.S., (2008), Learning about seasons in a technologically enhanced environment: The impact of teacher-guided and student-centered instructional approaches on the process of students' conceptual change, Science education, 92, 320–344.
- Hawkes S.J., (1992), Arrhenius confuses students, Journal of Chemical Education, 69, 542–543.
- Johnson D. W., Johnson R. and Smith K., (1991), Active learning: Cooperation in the college classroom, Edina, MN: Interaction Book Company.
- Johnson D. W. and Johnson R. T., (1994), Joining together: Group theory and group skills, Boston: Allyn and Bacon.
- Kaya O. N., (2007), A student-centred approach: Assessing the changes in prospective science teachers' conceptual understanding by concept mapping in a general chemistry laboratory, Research in Science Education, 38, 91–110.
- Lazarowitz R., Baird J. H., Hertz-Lazarowitz R. and Jenkins J., (1985), The effect of modified jigsaw on achievement, classroom social climate and self-esteem in high school science classes, in: Slavin, R. et al. (ed.) Learning to Cooperate, Cooperating to Learn, New York & London: Plenum Press, pp. 231–253.
- McKeachie, W. J., (1986), Teaching Tips, (8th edn) Lexington, Mass.: Heath.
- Nakhleh M.B. and Krajcik J. S., (1993). A proctocol analysis of the influence of technology on students' actions, verbal commentary, and thought processes during the performance of acid–base titrations, Journal of Research in Science Teaching, 30, 1149–1168.
- Nakhleh M. B., (1994), Students' models of matter in the context of acid–base chemistry, Journal of Chemical Education, 71, 495–499.
- Nakhleh M. B., (1992). Why some students don't learn chemistry, Journal of Chemical Education, 69, 191–196.
- Osborne R. and Freyberg P., (1985), Learning in science: The implications of children's science, Portsmouth, NH: Heinemann.
- Özmen H., (2003), Chemistry student teachers' levels of linking their knowledge with daily life about acid and base concepts, Kastamonu Education Journal, 11, 317–324.
- Peterson R.F., Treagust D.F. and Garnett P., (1989), Grade-12 students misconceptions of covalent bonding and structure, Journal of Chemical Education, 66, 459–460.
- Rayner-Canham G., (1994), Concept of acids and bases, Journal of College in Science Teaching, 23, 246–247.
- Ross B. and Munby H., (1991), Concept mapping and misconceptions: a study of high-school students' understandings of acids and bases, International Journal of Science Education, 13, 11–24.
- Sanger M. J., (2000), Addressing student misconceptions concerning electron flow in aqueous solutions with instruction including computer animations and conceptual change strategies, International Journal of Science Education, 22, 521–537.
- Schmidt H.J., (1991), A label as a hidden persuader: chemists' neutralization concept, International Journal of Science Education, 13, 459–472.
- Schmidt H. J., (1995), Applying the concept of conjugation to the Brønsted theory of acid–base reactions by senior high school students from Germany, International Journal of Science Education, 17, 733–741.
- Schmidt H. J., (1997), Students' misconceptions—looking for a pattern, Science Education, 81, 123–35.
- Sharan S. and Hertz-Lazarowitz R., (1980), A group-investigation method of cooperative learning in the classroom, Cooperation in Education, Provo, Utah: Brigham Young University Pre.
- Sheppard K., (1997), A qualitive study of high school students pre- and post-instructional conceptions in acid–base chemistry, PhD diss., Colombia University.
- Sheppard K., (2006), High school students' understanding of titrations and related acid–base phenomena, Chemical Education Research and Practice, 7, 32–45.
- Sisovic D. and Bojovic S., (2000), Approaching the Concepts of Acids and Bases by Cooperative Learning, Chemistry Education: Research and Practice in Europe, 1, 263–275.
- Slavin R.E., (1980), Cooperative learning, Review of Education Research, 50, 315–42.
- Smith K. J. and Metz P. A., (1996), Evaluating student understanding of solution chemistry through microscopic representations, Journal of Chemical Education, 73, 233–235.
- Taber K., (2000), Chemistry lessons for universities?: A review of constructivist ideas, University Chemistry Education, 4, 63–72.
- Toplis R., (1998), Ideas about acids and alkalis, School Science Review, 80, 67–70.
- Vidyapati T. J. and Seetharamappa J., (1995), Higher secondary school students' Concepts of acids and bases, School Science Review, 77, 82–84.
- Young W., Hadgraft R. and Young M., (1997), An application of ‘jigsaw learning’ to teaching Infrastructure model development, European Journal of Engineering Education, 22, 11–18.
- Zoller U., (1990), Students' misundestanding and misconceptions in college freshman chemistry (general and organic), Journal of Research in Science Teaching, 27, 1053–1065.
| This journal is © The Royal Society of Chemistry 2012 |
