On the nature and signatures of the solvated electron in water
B.
Abel
*a,
U.
Buck
b,
A. L.
Sobolewski
c and
W.
Domcke
d
aWilhelm-Ostwald-Institut für Physikalische und Theoretische Chemie, Universität Leipzig, D-04103 Leipzig, Germany. E-mail: abel@rz.uni-leipzig.de
bMax Planck Institut für Dynamik und Selbstorganisation, D-37073 Göttingen, Germany
cInstitute of Physics, Polish Academy of Sciences, PL-02668 Warsaw, Poland
dDepartment Chemie, Technische Universität München, D-85747 Garching, Germany
First published on 11th November 2011
Abstract
The hydrated electron is one of the simplest chemical transients and has been the subject of intense investigation and speculation since its discovery in 1962 by Hart and Boag. Although extensive kinetic and spectroscopic research on this species has been reported for many decades, its structure, i.e., the dominant electron–water binding motif, and its binding energy remained uncertain. A recent milestone in the research on the hydrated electron was the determination of its binding energy by liquid-jet photoelectron spectroscopy. It turned out that the assumption of a single electron binding motif in liquid water is an oversimplification. In addition to different isomers in cluster spectroscopy and different transient species of unknown structure in ultrafast experiments, long-lived hydrated electrons near the surface of liquid water have recently been discovered. The present article gives an account of recent work on the topic “solvated electrons” from the perspectives of cluster spectroscopy, condensed-phase spectroscopy, as well as theory. It highlights and critically discusses recent findings and their implications for our understanding of electron solvation in aqueous environments.
I. Introduction
The solvated electron is one of the simplest reactive species in chemistry and biology. Electrons trapped in liquid ammonia, water, alcohols, amines and other polar liquids have been of interest in chemistry since a long time. The hydrated electron, in particular, is a fundamental transient species in the radiation chemistry of water. Initially, it has been identified as a second reducing agent (besides the hydrogen atom) in the pulse radiolysis of pure water, which is formed with a higher yield than the H atom.1 The absorption spectrum of this species, centered at 720 nm, was discovered by Hart and Boag in 1962.2 This intense, broad and characteristically asymmetric absorption spectrum,3 which is shown in Fig. 1, has become the most characteristic feature of the hydrated electron.1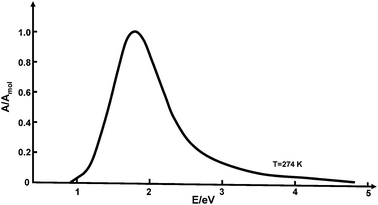 | ||
| Fig. 1 Absorption spectrum of the hydrated electron in bulk water under ambient conditions (adapted from ref. 113). | ||
Prominent features of the hydrated electron are its finite lifetime (of the order of microseconds in pure water), its exceptionally high rate of diffusion and its high reactivity.1 The kinetic rate constants of numerous reactions involving the hydrated electron have extensively been investigated and tabulated.4 Apart from its role in the radiation chemistry of water (e.g., in nuclear-reactor and nuclear-waste technology5), the hydrated electron is also formed by ionizing radiation in biological tissues and has a significant potential for the damage of DNA.6 From the fundamental point of view, the hydrated electron has fascinated generations of chemical physicists as the simplest possible model of a quantum particle interacting with a classical thermal environment.7–10
The present article is intended to give a timely account of recent developments in the investigation of the hydrated electron, in particular by time-resolved photoelectron emission spectroscopy, by the spectroscopy of size-selected aqueous clusters, as well as with computational methods. The paper is organized as follows: after a short general introduction we discuss theoretical models of the solvated electron in the condensed phase. Recent insights obtained by the spectroscopy of negatively charged water clusters, water clusters doped by alkali metal atoms, as well as acidic water clusters are surveyed in Section III. In Section IV, we summarize recent experiments on the photoelectron spectroscopy of hydrated electrons in liquid water beams that yield reliable binding energies of the solvated electron—one of the missing links in the investigation of the solvated electron in water. In Section V we critically discuss what is known and what is not yet known about this species. We close with conclusions and outlook.
II. Models of the structure and dynamics of the hydrated electron in the liquid phase
Many decades after its discovery, the hydrated electron is still not fully characterized in terms of structural and spectroscopic properties. The concept of an excess electron which is trapped in a cavity or void in the hydrogen-bonded network of liquid water was first proposed by Stein, Platzmann and Weiss already in the 1950s.7–9 The first attempts towards a quantitative description of the properties of the hydrated electron were based on the model of a spherical charge distribution interacting with a polarizable dielectric continuum.10Alternative to continuum models, so-called molecular-field models were proposed, where it is assumed that the excess electron is surrounded and solvated by a fixed number of water molecules in a specific configuration, e.g. in tetrahedral11 or octahedral12 cages. A proposed geometrical structure of the hydrated electron, derived from electron spin resonance data in aqueous glasses, is shown in Fig. 2. A comprehensive review of the early continuum and molecular-field models of the hydrated electron in bulk water was given by Feng and Kevan.13
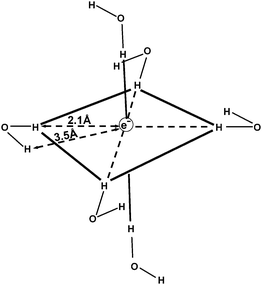 | ||
| Fig. 2 Proposed geometrical structure of the solvated electron in aqueous glasses, derived from electron spin resonance data (adapted from ref. 13). | ||
Since the 1980s, the so-called cavity model of the hydrated electron has been elaborated in considerable detail by Rossky, Borgis, Schwartz and coworkers, treating the excess electron as a localized quantum particle in the environment of classical rigid water molecules.14–18 Using partially empirical electron–water pseudopotentials, extensive numerical simulations of the stationary and transient absorption spectra of the hydrated electron were performed. While the position and width of the stationary absorption spectrum could be reproduced, the simulations failed to account for the characteristic asymmetry and the extended “blue tail” of the absorption profile (see Fig. 1).14–18 Very recently, it has been demonstrated that the blue tail can be described in simulations with electronically polarizable water molecules.19 On the other hand, a recent simulation employing an improved electron–water pseudopotential suggests a model in which the excess electron resides in a region of enhanced water density rather than in a cavity.20 Despite enormous progress in ab initio quantum chemistry and in the available computer power since the initial conception of the cavity model, a quantitative theory of the structure and the properties of the hydrated electron in bulk water is still elusive.19–21
While the cavity model has been the consensus picture of the hydrated electron since six decades, alternative proposals were sporadically made. In the 1980s, Robinson and collaborators as well as Tuttle and Golden called the cavity model into question and proposed that molecular solvent–anion complexes or radical–anion complexes, such as hydrated H3O–OH−, shown in Fig. 3, could be the carriers of the spectroscopic and chemical properties of the hydrated electron.22,23 Muguet and Robinson emphasized the itinerant character of the H3O radical in water which might explain the exceptionally high mobility of the hydrated electron.24 Sobolewski and Domcke pointed out (see also Section III) that the hydrated hydronium (H3O) radical is a charge-separated species in water and as such very effectively solvated,25–27 see also ref. 28. The calculated geometrical structures and the molecular orbitals of the unpaired electron of H3O(H2O)3n, n = 0, 1, 2, clusters are shown in Fig. 4. It is seen that the charge density of the unpaired electron detaches from the H3O+ cation with increasing cluster size. Moreover, the ab initio calculated electronic and vibrational spectra of the hydrated H3O radical exhibit striking similarities with the spectral signatures of the hydrated electron.25 For example, the blue tail of the absorption spectrum is readily explained by such molecular models of the hydrated electron.26,27
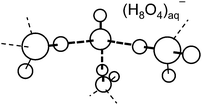 | ||
| Fig. 3 “Intuitive” structure, (H3O–OH−), of the hydrated electron in bulk water, postulated by Hameka, Robinson and Marsden (adapted from ref. 22). | ||
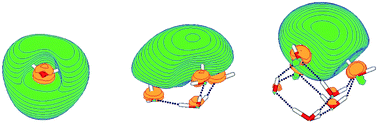 | ||
| Fig. 4 The singly occupied highest molecular orbital of H3O (left), H3O(H2O)3 (middle) and H3O(H2O)6 (right) clusters, calculated at the restricted open-shell Hartree–Fock level.25 The colors green/yellow code the sign of the wavefunction. Dotted lines indicate hydrogen bonds. | ||
In addition to pulse radiolysis with X-rays or electrons, the hydrated electron can be generated by single-photon or two-photon excitation of water,29–35 by photodetachment from anions in aqueous solution36–40 or by photoexcitation of organic chromophores with acidic groups, such as indole or phenol.41–46 The resonant photodetachment of electrons from anions in dilute salt solutions is the most convenient method of generation of hydrated electrons, since the absorption cross sections of anions in water are large, the excitation energy is convenient for lasers, and the quantum yields are high.40 Despite decades of intensive research, the mechanisms involved in photoionization in aqueous solutions remain controversial. It has been pointed out by Bradforth and coworkers that electron ejection from neat water and photodetachment of anions in water may be quite distinct processes.47 The mechanisms of relaxation towards the fully equilibrated hydrated electron are complex and depend strongly on the excitation process. In the case of anion photodetachment, it is assumed that charge-transfer-to-solvent (CTTS) states are transiently populated.40,47 In the case of UV two-photon excitation of neat water, a number of intermediate species (“wet electrons”) have been postulated for the interpretation of transient spectra.48 The existence of such intermediate species and their association with structural models remain controversial issues.49–53
The radiationless decay of the s → p excited state of the hydrated electron has been another hotly debated issue since several decades. The ongoing controversy concerns the mechanism by which the electron relaxes to equilibrium after impulsive photoexcitation. While the femtosecond pump–probe data obtained by several groups are similar, the controversies arise in the interpretation. It is disputed whether the relaxation occurs by rapid nonadiabatic radiationless decay to the electronic ground state, followed by comparatively slow (picosecond) ground-state cooling, or by relatively slow nonadiabatic excited-state decay, followed by rapid equilibration in the ground state.54–57 Quantum-classical molecular simulations can explain either scenario, depending on the electron–water pseudopotential and the nonadiabatic surface-hopping algorithm.16,20,58–61
III. The cluster approach: models of the structure and the dynamics of electrons in finite-size aqueous clusters
Already very early, experiments with clusters played a crucial role in elucidating the structure and the spectroscopic properties of the hydrated electron. Negatively charged water cluster anions are natural finite-size models of the hydrated electron. The size selection is no problem and once it was discovered how to produce these clusters,62,63 measurements of the binding energies of the excess electron were carried out for large64,65 and small clusters.66 The size dependence of the electron binding energy followed the predicted behaviour.67,68 Based on the picture of spherical clusters of radius R, a plot of the measured vertical binding energies versus 1/R (and thus n−1/3, where n is the cluster size) gives straight lines with negative slopes for the cluster anions. Theoretical estimates of the slope can be derived from dielectric continuum theory. An important issue, which is often overlooked in using this type of diagram, is the fact that this theory, which is based on bulk matter concepts, does not distinguish between surface and interior states of the electron.67 The extrapolation of the data for anionic water clusters to the bulk value (n → ∞), reported by the Bowen group,65,69,70 gives 3.3 eV. These data are shown in Fig. 5 in blue colour.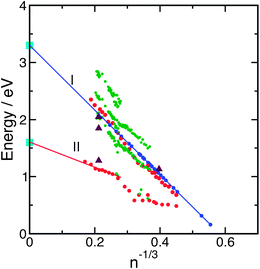 | ||
| Fig. 5 Vertical binding energies of (H2O)n−clusters (blue: ref. 65; red: ref. 74; green: ref. 81) as functions of n−1/3. The light blue points mark the binding energies of interior and surface electrons in the liquid.129 The violet triangles are based on recent calculations.144 | ||
The experimental results were accompanied by calculations which dealt with the structures and energetics of these clusters. For large clusters in the size range n = 8–128, the quantum path-integral molecular dynamics method with a pseudopotential for the electron–water interaction was used.71,72 The calculations predicted the existence of diffuse surface-bound states as well as more compact interior states of the excess electron. For small and intermediate sized clusters (n = 8–32), the energetically favoured localisation of the excess electron is a surface state, while for larger clusters (n = 64, 128), the internal localisation of the electron is preferred. The calculation of the electronic absorption spectra gave good agreement with the experimental data for the surface states.73
Recent extensive photoelectron emission and laser-spectroscopic investigations of intermediate-size and large water cluster anions by the groups of Bowen, Johnson, Zewail and Neumark revealed a considerably more complicated picture.69,74–78 A major breakthrough in this field was the discovery of new classes of cluster isomers by the Neumark group when the conditions of producing the clusters were changed.74,79,80 The photodetachment spectra obtained for cluster sizes n = 11–200, produced at normal pressures of 2.1 bar of the water–argon mixture, were similar to those obtained by Coe et al.65 When the backing pressure was increased to 4.8 bar, however, a much lower value of the vertical binding energy was obtained. The complete results are plotted in Fig. 5 in red colour. The two distinct groups of isomers are labelled I and II. These findings have been confirmed in an experiment with larger photon energy (4.7 eV instead of 3.1 eV).80 The explanation of these results is as follows. In the case of expansions at normal pressure (group I), the electrons are attached to relatively warm clusters with high internal energy, which readily leads to the lowest energy configurations by solvent rearrangement. The expansions at high pressure (group II), on the other hand, produce rather cold clusters with small reorganisation energies, which carry the excess electron in a metastable state, preferentially at the surface of the cluster. This interpretation was confirmed by a very recent measurement of the vertical binding energies of very cold clusters.81 In this experiment, heating occurred between the production and the thermalisation of the clusters to low temperatures, so that the clusters of class II are nearly completely suppressed and only isomers of class I are observed. The photoelectron spectra consist of up to three peaks, the energies of which are plotted in Fig. 5 in green colour. Very recent DFT-based molecular dynamics simulations for n = 32 demonstrated that in cold clusters the electron becomes trapped in a metastable state with comparatively low binding energy,82 while electron attachment to warm, liquid clusters results in an equilibrated and comparatively strongly bound species.83,84 Since calculations usually predict that internally solvated electrons are more strongly bound than those at the surface,71,85 the class I isomers are assigned to internally bound electrons and class II isomers to surface-bound electrons. We note, however, that the calculated binding energies do not agree with the measurements, being in both cases too large. Interestingly, the measured electronic absorption spectra86 are better reproduced by the surface isomers in the computational simulations up to n = 50.85 The experimental results were complemented by the measurement of electronic relaxation time scales75,76 and vibrational spectroscopy of the OH stretching band.77 In the latter case, the spectra exhibit the double-acceptor motif for small clusters of the isomer class I.87 From the variety of experimental results and the different data sets for the class I isomers, it is difficult to pin down exactly the group of clusters for which the data extrapolate correctly to the bulk values. Recent Born–Oppenheimer (BO) molecular-dynamics simulations for (H2O)32− at T = 350 K, based on DFT with the PBE functional, showed a strong correlation of the binding energy with the degree of localisation of the electron, rather than with the interior or surface character.88
Extensive simulations of (H2O)n−clusters of various sizes and at various temperatures with established mixed-quantum-classical methods did not lead to definitive structural assignments.85,89–91Ab initio molecular dynamics calculations provided evidence that the excess electron is located at the surface of the cluster, even if it is initially placed in the interior.88 Although numerous ab initio calculations with self-consistent-field (SCF), second-order Møller–Plesset (MP2) or DFT methods have been performed for selected structures of (H2O)n−clusters, it is not possible to explore systematically the isomers due to the huge number of possible structures.92–96 The question of the electron binding motif of intermediate-size water cluster anions and its extrapolation to bulk water thus remains unsettled.
An alternative approach towards the modeling of the hydrated electron by finite-size clusters is inspired by the well-known beautifully blue solution of metallic sodium in liquid ammonia.97 In the pioneering work of Schulz, Hertel and coworkers as well as Fuke and collaborators, the size-dependence of the vertical ionization energy, the vertical electronic excitation energy and the excited-state lifetime of M(H2O)n (M = Li, Na, K) clusters were determined.98–103 The electronic excitation energy of Na(H2O)n clusters exhibits a significant and non-monotonic dependence on the cluster size.101 The excited-state lifetimes of Na(H2O)n clusters are systematically shorter than those of (H2O)n−clusters.103 In IR spectra of size-selected sodium-doped water clusters, finger-print OH vibrations have been detected which indicate a delocalized electron at the surface of the clusters.104,105
The results of the Hertel group for the ionization potentials (IPs) of Na(H2O)n clusters were very surprising. Only the first four values of the measured IP decrease, as expected, in the n−1/3 plot, while the IP stays constant at a value of 3.17 eV for all larger clusters.99,100 Very similar results were obtained for Li(H2O)n clusters with an IP of 3.12 eV and Cs(H2O)n clusters with an IP of 3.10 eV for n ≥ 4.98,106 There were numerous speculations on the explanation of the unexpected constant behaviour of the IP of alkali–water clusters. In one of the first experimental papers,99 the transition from a one-center to a two-center localization of the electron was discussed, taking up an idea of Jortner.68 The earliest calculation on sodium–water clusters, based on spin density functional theory, confirmed indeed the complete separation of the electron from the Na+ cation from n ≥ 4 onwards and its delocalization over the water molecules.107 As for the reason of the constant ionization potential, a compensation of forces was suggested. Ab initio calculations at the UMP2 level were also carried out for small Na and Li water complexes.108,109 The calculated IPs for the interior complexes are lower than the experimental values, while the calculated IPs of the surface complexes are higher than the measured values. In a more recent publication, the constant IP was explained by an autoionization process after a transition to high Rydberg states and the release of considerable vibrational reorganisation energy.110
Very recently, a second class of isomers has been observed also for Na-doped water clusters in a special experimental set-up which is capable of generating molecular beams with high abundances of large clusters.111 These results are displayed in Fig. 6. One group with the already known IP of 3.2 eV (group I) has been extended up to the size n = 500, and the existence of a second group of isomers with the lower IP of 2.8 eV has been established (group II). In the second group, the IP decreases in the range 11 ≤ n ≤ 15 and then stays constant, as was found for class I. The existence of two classes of isomers in Na(H2O)n clusters calls for a comparison with the data obtained for the water cluster anions, which are included in Fig. 6. Apart from the different asymptotic behaviour, there are other remarkable differences. The bifurcation of the IPs into two branches occurs for the Na-doped clusters at smaller sizes and for the same expansion conditions. The difference of the two extrapolated electron binding energies is smaller for the Na(H2O)n clusters (0.4 eV) than for the largest (H2O)n−clusters (1.05 eV). For the Na(H2O)n clusters, the lower IP and the larger critical size of group II indicate that the charge-separation distance of the Na+–e− ion pair should be larger in the isomers II. This is indeed confirmed by a simulation of the structures, using ab initio molecular dynamics and the modelling of the ionization spectra with the PMP2 method.111 For n = 15, two isomers could be distinguished with IPs of 3.1 eV and 2.6 eV, respectively, close to the measured values. According to the simulations, the electron is in both cases distributed over the water molecules at the surface of the cluster. The isomers differ, however, in the Na+–e− distance, which is 3.28 Å for the group I clusters and 4.86 Å for the group II clusters. It was concluded that one isomer corresponds to a contact ion–electron pair and the other isomer to a solvent-separated electron–ion pair.111
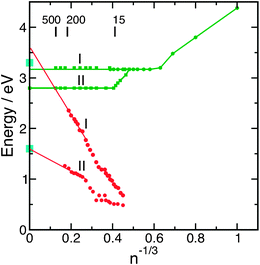 | ||
| Fig. 6 Ionisation potentials and vertical electron binding energies of the different isomers of Na(H2O)n clusters (green)99,111 and (H2O)n−clusters (red)74 as functions of n−1/3. The light blue points mark the binding energies of interior and surface electrons in the liquid.129 | ||
The hydronium (oxonium) radical, H3O, is isoelectronic with the sodium atom. It is thus not surprising that the H3O radical dissociates into an H3O+ cation and a solvated electron in water clusters,25,28,112 as has been found for alkali–water clusters. There is a fundamental difference, however: while the spherical alkali cations can be solvated by numerous conformations of the hydrogen-bonded network of water, the H3O+ cation is a rather rigid trigonal pyramid which forms exceptionally strong and directed hydrogen bonds with the first solvation shell (the so-called Eigen structure of protonated water). The H3O+ cation thus induces, by its rigid geometry, a microcavity in the hydrogen-bond network of water.25 It has been argued that the structural rigidity of the (H3O+)aq(e−)aq ion pair could be the origin of the surprising shape stability113 of the absorption spectrum of the hydrated electron.
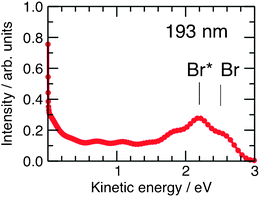
While H3O(H2O)n clusters are difficult to prepare directly (through charge exchange of H3O+(H2O)n clusters with alkali atoms114,115), their transient existence has been inferred indirectly through the photodissociation of hydrogen halide (HX) molecules in water/ice nanoparticles.116–118 The solvated H3O radical is generated in these experiments through CTTS excitation of the charge-separated H3O+X− species and is detected by the characteristic translational energy distribution of the H-atoms arising from the dissociation of metastable H3O(H2O)n clusters. The experiments on the photodissociation of pure and doped water clusters were carried out in the standard molecular beam arrangement.119 The HX(H2O)n clusters were generated by adiabatic expansion and then dissociated by UV laser photons at 193 nm or 243 nm. The arising H-atoms were observed by resonance-enhanced multiphoton ionisation at 243 nm. The H-atom fragment kinetic energy was detected in a time-of-flight spectrometer and is the main observable in these experiments. The photodissociation of hydrogen halides on Arnclusters, in which no ion-pair separation takes place, serves as a reference.120,121 The results for HClArnclusters are shown in Fig. 7.120 The narrow peak at zero kinetic energy corresponds to the caged fragments, while the peaks at higher energy result from the direct cage exit of the fragments, reflecting the two spin–orbit states of the Br radical. The results for the HX(H2O)n systems look completely different, as shown in Fig. 8.117,122 They exhibit only slow fragments with energies below 0.5 eV. The differences between the HXArn and HX(H2O)n kinetic-energy spectra can be explained by postulating the formation of the H3O radical in the HX(H2O)n clusters. The suggested mechanism is depicted in Fig. 9.117,118,122 It starts with the acidic dissociation of the HX molecule in the ground state, followed by laser excitation of the X− anion to a CTTS state. The latter relaxes by a radiationless transition to a H3OX biradical.94,122,123 The H3O radical is metastable and will ultimately decay, yielding an H2O molecule and the H atom which is finally detected. Several experimental findings support this picture.
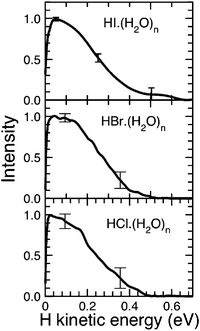 | ||
| Fig. 8 Kinetic-energy distribution spectra for the photolysis of HX(H2O)n clusters with n = 400.117,122 | ||
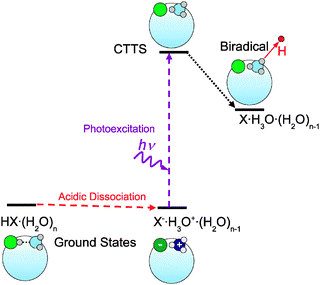 | ||
| Fig. 9 Mechanism of the photodissociation of HX(H2O)n clusters.117,118,122 | ||
(1) Double isotope substitution: measurements with isotopic variants of HX and H2O provide strong experimental evidence for the hypothesis that the detected H-atom originates from the H3O species. Assuming hydronium radical generation from the HX and H2O molecules (without H/D scrambling), the H3O, H2DO and HD2O radicals would be produced in HX(H2O)n, DX(H2O)n and HX(D2O)n clusters, respectively. Therefore, the expected ratio of H-signals from these species would correspond to the number of H-atoms in these radicals, i.e., 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.117,118Table 1 summarizes the measured ratios of the H-fragment signals from HX(H2O)n clusters to H-signals from the deuterated species, HD2O from HX(D2O)n and H2DO from DX(H2O)n, clusters, which are generally in good agreement with the expected values. These ratios suggest that statistical H/D scrambling does not occur in the clusters at least on the timescale of the present experiment (∼0.65 ms).
1.117,118Table 1 summarizes the measured ratios of the H-fragment signals from HX(H2O)n clusters to H-signals from the deuterated species, HD2O from HX(D2O)n and H2DO from DX(H2O)n, clusters, which are generally in good agreement with the expected values. These ratios suggest that statistical H/D scrambling does not occur in the clusters at least on the timescale of the present experiment (∼0.65 ms).
| X | Cl | Br | I | I | Expected |
|---|---|---|---|---|---|
| Wavelength, λ | 193 | 193 | 193 | 243 | |
| HX(D2O)n | 2.7 ± 0.4 | 3.0 ± 0.3 | 3.1 ± 0.5 | 3.9 ± 1.0 | 3.0 |
| DX(H2O)n | 1.1 ± 0.4 | 1.4 ± 0.2 | 1.6 ± 0.4 | 2.5 ± 1.0 | 1.5 |
(2) Shape of the H-atom kinetic-energy distribution: it has been mentioned above that the differences between the HXArn and HX(H2O)n kinetic-energy spectra indicate a mechanism of H-fragment formation in HX(H2O)n clusters which differs from the direct photodissociation of the HX molecule. In addition, the spectra in Fig. 8 are the same for all three HX molecules within the experimental error bars, which is not at all the case for the corresponding HXArnclusters. The similarity of these spectra suggests that the H-fragments originate from the same species in all three systems, namely from the H3O radical, rather than from the different HX molecules. The measured shape agrees well with what is known of the energetics of the decay H3O → H2O + H. The corresponding potential-energy curve is shown in Fig. 10.25 From the top of the low barrier, the maximal energy gain is about 0.7 eV, in good agreement with the measurements.
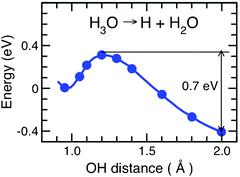 | ||
| Fig. 10 Potential-energy function along the minimum-energy path for H-atom detachment from the hydronium radical solvated by three water molecules, H3O(H2O)3 (adapted from ref. 25). | ||
(3) HCl/HBr cross-section ratio: the acidic dissociation of HCl and HBr leads to a significant red shift of the absorption spectrum. In fact, a new band, the CTTS band, appears. This band can be used to detect whether HCl or HBr are acidically dissociated under the experimental conditions. The ratio j(HBr)/j(HCl) of the photolysis rates at the specific wavelengths of 191 and 243 nm is 7.4 ± 1 for HX deposited on water clusters. Calculations for small clusters give values around 2 for the dissociated structures and about 45 for the covalently bonded structures.116 This is a clear indication that the HX molecules are acidically dissociated, preferentially forming a contact ion pair, since otherwise we would have observed a pronounced dilution in the isotopically substituted experiments. This result is in agreement with other experiments using Fourier transform IR-spectroscopy124 and Cs+ ion scattering125 from ice particles. The transition occurs at temperatures between 80 and 120 K, while the internal temperature of our clusters is estimated to be 100–120 K.126
Alternative evidence of the formation of hydronium–halogen biradicals by CTTS excitation has been obtained by Castleman and coworkers through the detection of the released iodine atom from HI(H2O)n clusters.127,128 Both experiments, the detection of the released hydrogen atom and the detection of the halogen atom, illustrate that cluster experiments can provide information through observables which are not easily accessible in the condensed phase, namely the kinetic-energy distribution of the nascent products. The experimental evidence of the central role of the H3O radical in the photochemistry of acidic and salty water clusters has been supported by computational studies of excited-state potential-energy surfaces of HX(H2O)n, X = Cl, Br and MX(H2O)n, M = Li, Na, K, clusters.94,123 There is thus increasing evidence that the hydrated H3O radical actually exists and that it can be detected spectroscopically—at least in (small and cold) water cluster environments. It is less clear whether it is actually the second reducing species in the radiolysis and photolysis of liquid water, as has been postulated by Robinson and coworkers long ago.22
IV. Photoelectron spectroscopy of hydrated electrons in liquid jets: the missing link
Very recently four research teams reported the determination of the vertical binding energy of the solvated electron in water, by liquid water microjet photoelectron spectroscopy.129–132 In all these experiments, the hydrated electrons were generated by resonant photodetachment of electrons from an anion (I− or Fe(CN)64−).40,133 In the first report on solvated electrons in a water micro-jet, Siefermann et al. additionally generated hydrated electrons by 267 nm two-photon excitation of neat liquid water.129 In ref. 130 and 131, the solvated electron was detected via photoemission after excitation with 274, 266, 260 or 213 nm laser light, while EUV radiation (32 nm) was employed in ref. 129. Recently, Lübcke et al. and Tang et al. reported femtosecond time-resolved photoelectron spectra of the hydrated electron in a liquid jet.132,134Instead of using UV photoelectron detachment, Siefermann et al. directly measured vertical binding energies of hydrated electrons in a liquid micro jet in vacuum employing a high-harmonic light source driven by a femtosecond laser system.135,136 The special features in their novel approach are the generation of solvated electrons by a femtosecond pump pulse and the registration of the photoelectron spectra using a time-delayed 38.7 eV (32 nm) probe pulse, using a multiplex photoelectron time-of-flight spectrometer to record the entire photoemission spectrum for a particular time step.
In the latter study, hydrated electrons in liquid water were generated first by 267 nm laser pulse excitation of Fe(CN)64− anions in a 0.5 M aqueous solution of K4Fe(CN)6. The (Fe(CN)64−)aq anion complex has a strong CTTS absorption band near 267 nm. After absorption of UV light, the released excited electron relaxes to an equilibrated solvated electron within approximately 0.5 ps.37 In the experiments, the EUV probe pulse was delayed by >100 ps relative to the CTTS excitation in order to assure equilibration of the electron. The intensity of the UV pump pulse was tuned to avoid two-photon excitation of water, but was sufficiently high to generate a significant density of hydrated electrons via CTTS excitation of Fe(CN)64− anions. The EUV photoelectron probe has a high surface sensitivity and selectivity. However, in this particular experiment the detected solvated electrons were generated mainly in the bulk and not at the surface, because Fe(CN)64− anions are known to be repelled from the surface.137
The photoelectron spectrum obtained from such an experiment is displayed in Fig. 11 (error bars are indicated). The strongly rising photoelectron signal near 4.5 eV results from the direct ionization of the ferrocyanide anion. The photoemission line peaking at 3.3 eV with a linewidth of 0.8 eV is observed only in the presence of the pump pulse. The photoelectron spectrum in Fig. 11 is the first direct measurement of the binding energy of a bulk hydrated electron in liquid water.
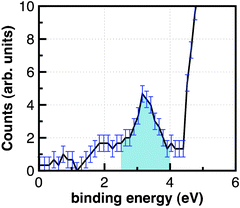 | ||
| Fig. 11 Photoelectron signal of the interior solvated electron in the liquid jet.129 | ||
A number of experiments by other groups using liquid-jet technology, but employing UV light (267–213 nm) instead of EUV light for ionization, have been reported recently as well. In these experiments, the hydrated electrons were generated also by CTTS excitation of anions (Fe(CN)64− or I−). From experiments of Tang et al.,130 a vertical electron binding energy of 3.27 eV was concluded, whereas Shreve et al.131 and Lübcke and co-workers132 measured 3.6 eV and 3.4 eV, respectively. Small deviations on the order of 0.1–0.2 eV in the measured binding energies of the hydrated electrons may arise from different anions as electron sources, different salt concentrations, or different (not perfect) calibration conditions or beam charging. However, from all available experiments an average reference value (3.4 ± 0.2 eV) for the ionization potential of the hydrated electron in liquid water can be deduced. As noted above, an additional source of error may arise from the calibration procedures used in ref. 130–132 which are based upon gas-phase measurements rather than on liquid-phase measurements. Shreve et al.,131 who generated hydrated electrons from (I−)aq and (Fe(CN)64−)aq, could show that the binding energy of (e−)aq is insensitive to the choice of the precursor. The experiments by Tang et al.130 and Lübcke et al.132 additionally revealed some new information on the solvation dynamics following CTTS excitation of I− in aqueous solution and provided a somewhat more complete picture of the ultrafast ejection, solvation and recombination dynamics of the hydrated electron.
In an additional experiment by Siefermann et al., liquid water in a micro-jet was excited with two 267 nm photons, corresponding to an excitation energy of 9.30 eV, just below the ionization potential of liquid water.29–31 The measured EUV photoelectron emission spectrum is displayed in Fig. 12. Contrary to the spectrum in Fig. 11, the photoemission peaks at 1.6 eV binding energy (with a somewhat larger width of 1.3 eV) appear—as in the previous case—only in the presence of the 267 nm pump pulse. This spectrum, which has been generated by two-photon excitation of water, was assigned to a novel type of hydrated electron. Surprisingly, identical pump–probe photoelectron spectra were obtained when the delay time of the EUV probe pulse was varied between 2 and 100 ps, which implies that the lifetime of this transient, which was finally assigned to a surface-bound hydrated electron by Siefermann et al., exceeds 100 ps.
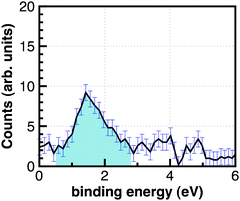 | ||
| Fig. 12 Photoelectron signal of the surface-bound electron in the liquid jet.129 | ||
A photoemission signal of the less strongly bound hydrated electron (1.6 eV binding energy) could only be detected by UV two-photon excitation of neat water and with EUV ionization. The assignment of the transient to correspond to a surface-bound electron is consistent with the very low probing depth of EUV generated electrons.138,139 The differences to the experiments not observing the surface-bound electron are the precursor and the probe wavelength. With respect to the latter, it is important to realize that the photoemitted electrons generated by the EUV probe pulse possess kinetic energy of about 35 eV, which is near the minimum of the electron attenuation length (EAL) curve of about 2 μm,138,139 making the ultrafast probe very surface selective for short-lived species. In the laser photoionization experiments of Tang et al., Shreve et al., and Lübcke et al., on the other hand, the photoelectron kinetic energies are of the order of 1 eV, for which the EAL is at least one order of magnitude larger, which explains why the surface-bound electron could not be detected, although Lübcke et al. report an excellent dynamical range of their set-up (≫1000). Another reason may be the choice of the different precursors, which may be an indication that this surface species can be generated by two-photon excitation of water, but not by laser photodetachment of solvated anions. This conjecture is supported by the fact that another surface-sensitive technique, second harmonic generation (SHG), also failed to detect a surface-bound electron when applied to hydrated electrons generated by CTTS excitation of I− anions.140 The hydrated electrons prepared by anion photodetachment in salt solutions and by two-photon UV excitation of neat water thus may be physically or chemically distinct species. Recently, the Mafune group reported spectra of iodide anions hydrated at the surface and in the bulk, respectively. It was pointed out that excitation with different wavelengths (and thus excitation of different CTTS bands) may produce different transients of the hydrated electron.141
Shedding light on the energetics and qualitative binding motifs of the bulk hydrated electron, (e−)aq, and the surface-bound hydrated electron, (e−)sf, is quite important for the understanding of how electrons can attach to molecules in aqueous environments and break covalent bonds. A cartoon picture of a typical bond-breaking situation for an organic molecule is displayed in Fig. 13. On the left-hand side, the binding energy (ruler) of the solvated electron and its photoemission spectrum are shown. As has been discussed in detail in ref. 129, the energy acceptance window of organic molecules with energetically suitable LUMOs (lowest unoccupied orbitals) for solvated electrons is governed by the energies for vertical electron attachment (VEA), adiabatic electron attachment (AEA), as well as the vertical electron detachment energy (VDE) of the target molecules. As can be seen in Fig. 13, the surface-bound solvated electron is often approximately isoenergetic with the electron attachment energy of many organic molecules, such as DNA or CFCs (chlorinated and/or fluorinated hydrocarbons), for example. Thus, the measured binding energies of solvated electrons provide reference values for important electron-transfer processes taking place in aqueous solution, in particular resonant dissociative electron attachment (RDEA) processes. The VBEs of (e−)aq and (e−)sf together with the RDEA model allow us to understand how electrons in aqueous solution can easily break strong covalent bonds in systems like DNA or CFCs. This qualitative picture reveals (in a semiquantitative fashion) why (e−)sf as well as optically excited hydrated electrons may play an important role in radiation-induced DNA damage and organic molecule bond cleavage. RDEA of organic molecules like CFCs indicates that the (e−)sf transient may also be involved in atmospheric ozone chemistry, although the main reaction channels are dominated by radicals rather than ionic species.129
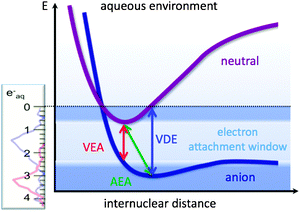 | ||
| Fig. 13 Schematic view of the energy acceptance window for solvated electrons depending upon the VEA (vertical electron attachment energy), the VDE (vertical detachment energy), and the AEA (adiabatic electron attachment energy).129 | ||
V. Synopsis: what do we know about the solvated electron in clusters, at interfaces and in the bulk
The measured binding energies of the hydrated electron prepared by laser photodetachment from anions in a liquid jet are 3.3 eV,129,130 3.4 eV,132 and 3.6 eV,131 which lead to a not very accurate, but reliable reference value (3.4 ± 0.2 eV) for the ionization potential of the hydrated electron in liquid water. A more weakly bound electron (1.6 eV binding energy), prepared by UV two-photon excitation of neat water, could be detected only with EUV ionization.129 The interpretation as a surface-bound hydrated electron is consistent with the very low probing depth of EUV generated electrons.129 The existence of a long-lived (>100 ps) surface-bound electron in liquid water with an ionization potential of 1.6 ± 0.1 eV has thus been established for the first time. While surface-bound hydrated electrons with low electron binding energies have frequently been observed in cold anionic water clusters, this is the first report of a surface-type electron in water at ambient temperatures. The observation of two significantly different ionization potentials of the solvated electron in liquid water is a surprising and important finding in the long and entangled history of the hydrated electron. The notion of a single equilibrated hydrated electron species in liquid water, which pervades all previous experimental and theoretical research in the condensed phase, seems to be an oversimplification.It should also be kept in mind that the two species with ∼3.4 eV and 1.6 eV binding energies, respectively, were prepared in a different manner. The hydrated electron with the larger binding energy was generated by a CTTS transition in a salt solution.129–132 In this case, the solvated excess electron is accompanied by a positive counterion (Na+ or K+ in the experiments discussed here). It is plausible that the positive counter-ion stabilizes a rather localized electronic charge distribution in the interior of the solvent, which can explain the larger binding energy of the bulk hydrated electron compared to the surface-bound hydrated electron. Calculations predict that the electronic absorption spectrum and the binding energy of the solvated electron are essentially independent of the counter-ion (Li+, Na+, K+, etc. in the case of salt solutions, or H3O+ in the case of neat water or acidic solutions).27,142
The surface-bound electron in liquid water was generated by excitation of neat water with two 267 nm photons.129 The excitation energy of 9.30 eV is below the ionization potential of liquid water.40 A water molecule is thus excited to a high Rydberg state. If the excitation occurs sufficiently close to the surface, the diffuse Rydberg orbital is “squeezed” out of the tight hydrogen-bond network of water, whereas the H2O+ cation is stabilized (at least for very short times) by solvation within the liquid. The measured binding energy of the surface-bound electron in water129 is consistent with the binding energy of surface-type hydrated electrons in cold (H2O)n−clusters.79–81 The bulk solvated and the surface-bound electrons may thus be distinct (chemical) species (a (M+)aq (e−)aq contact ion pair in the former case, and a separated (H2O+)aq (e−)aq pair in the latter case). This concept can explain the long lifetime of the surface-bound electron in liquid water. Otherwise, the existence of a substantial barrier for the incorporation of the surface electron into the bulk has to be assumed. Such a barrier seems unlikely in warm, liquid water.82–84
With the knowledge of definitive values of the ionization potentials of surface and interior excess electrons in liquid water at room temperature, we may return to the analysis of the extrapolation of the (H2O)n− and M(H2O)n cluster data towards n = ∞ (cf. Section III). The early data set65 for the group I water anions extrapolates nicely to the newly established ionization potential of the bulk hydrated electron, see Fig. 5. The more recent data for larger clusters, on the other hand, appear to extrapolate to an ionization potential of 3.6 eV74 or 4.0 eV,81 somewhat larger than the bulk value, see Fig. 5. This finding is surprising in view of the lack of a positive counter-ion in these clusters. Could it be that the excess electron induces ionic dissociation of a water molecule in large clusters, forming (H3O+)aq, (e−)aq and (OH−)aq? The less effectively screened interaction of (H3O+)aq and (e−)aq in finite-size clusters could explain the unexpectedly high value of the ionization potential of (e−)aq in (H2O)n−clusters. The water cluster anions of group II exhibit smaller electron binding energies which extrapolate nicely to the newly determined value (1.6 eV) of the surface-bound hydrated electron when only the cluster data for the larger sizes are taken into account (see Fig. 5).74 This fact strongly supports the assignment of the group II clusters as surface-bound anions. This interpretation implies that the binding energy of the surface-bound electron in the liquid jet is not affected too much by the presence of the H2O+ counter-ion.
The deciphering of the electronic and molecular structures of the multiple isomers of large water cluster anions may not be possible without predictive theory. An important step forward has recently been achieved by the calculation of electron binding energies of large (H2O)n−clusters with ab initio methods for all electrons.143,144 The results obtained for (H2O)−104 are included in Fig. 5 as violet triangles. The explicit values of the binding energies of (H2O)−104 are 2.05 eV for the compact (interior) structure and 1.28 eV for the diffuse (surface) structure.144 The agreement with the experimental data is very satisfactory. Interestingly, the calculation predicts also a compact surface state with a binding energy of 1.84 eV, which does not fall into either group I or group II and which has not been detected yet. According to these calculations, the binding energy is less correlated with the location of the excess electron on the surface or in the interior but with the compactness of the electron density distribution. Large binding energies correspond to compact and small binding energies to diffuse electron distributions. This correlation has been suggested previously by calculations for smaller cluster sizes of n = 32 as well.88 In addition, the same authors found that the process of electron attachment to a neutral water system and subsequent localization is quite different for ambient and cryogenic conditions.82,84 In the former system, the cluster quickly reaches an equilibrated structure, which corresponds to a well localized and strongly bound solvated electron. In contrast, in a cold system the electron gets trapped in a metastable “cushion like” state at the periphery of the cluster, which is more weakly bound. This rationalizes the observation of several isomers in different cluster environments. It is plausible that anionic clusters with a diffuse electron distribution extrapolate to the weakly bound diffuse state of the liquid (surface state), while the clusters with a compact electron distribution correlate with the compact distribution in the liquid (interior state). We note that despite of the fact that the clusters are solid-like and the liquid is fluid, this extrapolation is justified, since the properties of the solvated electron are dominated by the different shapes of the electron density and not so much by the solid/liquid phase of the aqueous environment.
A common feature of the solvated electrons prepared by laser photodetachment of salt anions (X−) in bulk water and by dissolution of alkali atoms (M) in finite-size water clusters is the presence of a positive counter-ion. The neutral X˙, which is generated by the photodetachment of X−, interacts weakly with the electron and the solvent and apparently does not affect the electronic spectra and the electron binding energy. If the escape length of the electron is large enough and if it does not recombine via geminate recombination with the precursor, we may regard it (and its binding energy) to be unperturbed by the counter ion.
It is nevertheless interesting to compare the electron binding energies of neutral M(H2O)n clusters with the electron binding energy of the bulk solvated electron in the liquid jet. They should only be equal if the electron in the former case is sufficiently far away or unperturbed from the cation, i.e., in sufficiently large water clusters. The extrapolation of the ionization potential of the most prominent group I of sodium–water clusters yields 3.2 eV (see Fig. 6), in good agreement with the ionization potential of the hydrated electron photodetached from Fe(CN)64− anions in the liquid jet (3.3 eV). Simulations of the electronic structure of M(H2O)n clusters indicate that the electron distribution is localized and corresponds to an electron–ion contact pair configuration.111 The ionization potentials of the group II of Na(H2O)n clusters extrapolate towards 2.8 eV, see Fig. 6. A corresponding species in the bulk has not been detected so far.
A comprehensive review of the properties of the solvated electron in other solvents than water is beyond the scope of this perspective. However, a brief comparison of the extrapolation of the aqueous cluster ionization potential towards the bulk limit with the corresponding data for two other representative solvents, ammonia and methanol, is illuminating. The data available for (NH3)n−clusters and Na(NH3)n clusters are shown in Fig. 14.145 The ionization potentials of Na(NH3)n clusters decrease approximately linearly with n−1/3, while the electron detachment energies of the (NH3)n− anions increase linearly with n−1/3. We note that the curves in these plots reproduce the measured points very well. They are calculated based on the continuum theory. Here it is crucial that the static and the optical dielectric constants of the solid material are used, in accordance with the probable thermodynamic state of the clusters under the specific production conditions. However, neither extrapolation seems to lead to the ionization potential of the solvated electron in bulk ammonia, see Fig. 14.146,147 The deviations can be explained by the presence of the counter-ion in Na(NH3)n clusters or the distance between them and the solvated electron which is larger in the liquid than in the solid clusters.
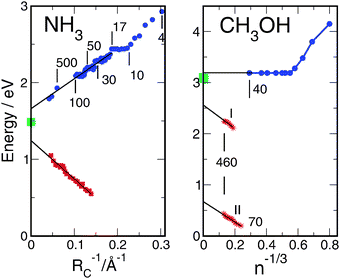 | ||
| Fig. 14 Ionization potentials and vertical electron detachment energies for ammonia145 and methanol149,150 as a function of the cluster size. Blue points: ionization potentials of neutral Na-doped clusters. Red points: detachment energies of negatively charged clusters. Green points: measured electron binding energies of the liquid.146–148 | ||
The results for methanol are similar, with the exception that the ionization potentials of Na-doped clusters are constant for large n. The ionization potential of the solvated electron in liquid methanol has very recently been determined in a liquid jet by laser photoionization of a NaI solution.148 Data for Na(CH3OH)n clusters149 and (CH3OH)n−clusters150 also have been reported recently. The scaling of the ionization potentials of the clusters with n−1/3 and the value of the bulk ionization potential (3.1 ± 0.1 eV) are displayed in Fig. 14. Two groups (I, II) of cluster anions with large and small electron detachment energies, respectively, have been observed. In both cases, the electron detachment energies scale linearly with n−1/3, see Fig. 14. As in the case of ammonia, the measured points are reproduced by calculations with the exception of the constant part of the ionization potentials of Na(CH3OH)n clusters which show a behavior which is reminiscent of water. After an initial decrease of up to n = 6, the ionization potential remains constant and extrapolates towards 3.2 eV, in good agreement with the measured bulk value, see Fig. 14. The electron detachment energies of the anions, on the other hand, extrapolate to electron binding energies which are significantly lower than the binding energy of the bulk solvated electron. As observed in ammonia, a stabilizing interaction with the Na+ counter-ion exists in the limit n → ∞. The solvated electrons with and without positive counter-ions are thus different species in methanol as well as in ammonia. We note that problems with the extrapolation of excess electron properties of amorphous solid clusters to the liquid bulk have been predicted earlier.82,84
In the comparison of the three solvents water, ammonia and methanol illustrated in Fig. 5, 6 and 14, the data for water are clearly exceptional and special. The ionization potentials of the group-I cluster anions extrapolate towards the ionization potential of the bulk solvated electron. The ionization potentials of the group-I Na(H2O)n clusters seem to extrapolate to the same value within the accuracy of the measurements, see Fig. 6. The ionization potential of an excess electron in large water clusters is thus not affected by the presence of a positive counter-ion, in contrast to ammonia and methanol clusters. The surface-bound electron in large (H2O)n−clusters extrapolates linearly in n−1/3 towards the surface-bound Rydberg state observed in the liquid jet. Again, the electron binding energy is not affected by the presence of a H2O+ counter ion in the case of neat water. Ammonia, methanol, and water are all hydrogen bonded systems. For water and methanol the binding energy of the hydrogen bond is significantly larger than for ammonia. The structure of water is dominated by a four-fold coordination, while methanol is arranged in linear long chains. This leads to strong short-ranged interactions in water relative to which counterions play a minor role. Another exceptional feature of water is its incipient ionic dissociation in very large clusters and in the bulk. As a result, excess electrons may induce H3O+ counter-ions in neat water, an effect which blurs the distinction between clusters with and without positive counter-ions.
An open question which has found relatively little attention in the literature is the origin of the finite lifetime of the equilibrated solvated electron, which is believed to be of the order of microseconds in neat liquid water.1 In photoexcitation of HX(H2O)n clusters, clear evidence for the formation of metastable H3O(H2O)n clusters has been found, but the lifetime of these species was too short to be measured in these experiments.117,122,127 By extension of the time delay in liquid-jet photoionization measurements beyond nanoseconds, it may be possible to measure the lifetime of the hydrated electron in the electronic ground state under well-controlled conditions.
VI. Conclusions and outlook
We have surveyed the currently available knowledge on the binding energies of solvated electrons in various size-selected aqueous clusters as well as in the liquid water jet. In liquid water, the existence of two solvated electron species with binding energies of ∼3.4 eV and ∼1.6 eV has been established so far. The species with 3.4 eV binding energy is tentatively identified as the equilibrated solvated electron in water under ambient conditions with a compact electron distribution. This assignment is tentative insofar as the optical absorption spectrum of this species, which is the defining property of the hydrated electron, has not yet been measured. The species with the lower binding energy (1.6 eV), which could be detected only with EUV ionization and with two-photon excitation of water as a precursor, has tentatively been assigned as a surface-bound hydrated electron in liquid water. The lower binding energy indicates a more extended electron-density distribution of this species. Since the interior electron and the surface-bound electron have been prepared in different manners (by CTTS excitation of a salt solution and by two-photon UV excitation of neat water, respectively), they could be chemically distinct species. The interior electron prepared in salt solutions may be associated with an alkali counter-ion, while the equilibrated surface-bound electron prepared in neat water presumably is separated from its original H2O+ counter-ion.We have summarized and discussed the large amount of electron binding-energy data which are available for (H2O)n− and Na(H2O)n clusters of various sizes and temperatures. In the plot of these electron binding energies vs. n−1/3, certain groups of isomers can readily be identified. The binding energies of two of these groups seem to extrapolate towards the two binding energies measured in the liquid jet. The data are consistent insofar as the binding energies of a group of clusters with an internally solvated electron mainly extrapolate towards the binding energy of the internally solvated electron in the liquid (3.4 eV), while the binding energies of a group of clusters carrying a relatively weakly bound surface-type electron extrapolate towards the binding energy of the surface-bound electron in the liquid (1.6 eV). In addition, they also agree in the type of the electron distribution, that is, compact for the internally bound electron and dispersed for the surface bound electron. We note, however, that there exist isomers of (H2O)n− as well as of Na(H2O)n clusters which do not have an analogue in the liquid, e.g., a group of (H2O)n−clusters with rather large binding energies, which seem to extrapolate towards 4.0 eV, or a group of Na(H2O)n clusters with a size-independent binding energy of ∼2.8 eV (see Fig. 5 and 6).
The comparison of the electron binding energies of anionic or alkali-doped clusters of water, ammonia and methanol clearly reveals the “anomalous” solvation properties of water. For ammonia and methanol, the extrapolated binding energies of clusters carrying an excess electron are significantly lower than the corresponding binding energies of alkali-doped clusters, revealing the stabilizing effect of the positive counter-ion in the alkali-doped clusters, see Fig. 14. With the exception of alkali-doped methanol clusters, the extrapolated binding energies of these clusters differ from the corresponding binding energy of the liquid. In the case of water, on the other hand, there exist clusters with internally bound electrons as well as with surface-bound electrons with ionization potentials which extrapolate towards the corresponding ionization potentials of the liquid, see Fig. 5 and 6. The electron binding energies of aqueous clusters seem to be determined by specific and relatively short-ranged hydrogen bonding interactions, rather than long-range dielectric screening effects. An alternative explanation of the anomalous solvation phenomena in large water clusters as well as in liquid water could be the unique tendency of water towards ionic dissociation. An excess electron in water may thus generate its own H3O+ counter-ion, resulting in an overall neutral (H3O+)aq (e−)aq solvated ion pair and an (OH−)aq anion.
The experiments on the photodissociation of hydrogen-halide doped water clusters, HX(H2O)n, have provided strong evidence of the existence of the hydronium radical, H3O, in the radiation chemistry of aqueous clusters. The HX molecules are acidically dissociated at the surface of water clusters (as well as in the liquid). When photoexcited to a CTTS state, they relax to an XH3O(H2O)n−1 biradical. Both the H3O radical as well as the X radical have definitively been detected in cluster experiments, the former through its decay to H2O + H and the characteristic kinetic-energy distribution of the released H atoms. For the liquid phase, such a direct proof of the relevance of the H3O radical is lacking. Calculations indicate, however, that the H3O radical is effectively solvated as a (H3O+)aq (e−)aq ion pair and that this ion pair carries the characteristic spectroscopic properties of the bulk hydrated electron.25,28 The hydronium cation, H3O+, may thus be the counter-ion of the bulk hydrated electron under certain circumstances.
The recent successful preparation and time-resolved detection of the hydrated electron in liquid water jets viaphotoemission spectroscopy—especially with extreme UV radiation—opens up new opportunities and avenues for the investigation of the structure and the dynamics of this elusive species, in particular at the interface of water due to the surface selectivity of EUV photoelectron spectroscopy. Nevertheless, even more than 50 years after the discovery of the hydrated electron, its dynamics, binding motifs and energetics are still not as well known as is desirable for the simplest of all chemical species in aqueous solution. Fundamental questions which remain to be answered are: (i) Can the interior hydrated electron be generated in the liquid jet by two-photon UV excitation of neat water? (ii) What happens when the excitation/generation wavelength is tuned over an extended range? (iii) Can we obtain insight into RDEA processes with hydrated electrons as intermediates with time-resolved experiments?
As demonstrated in ref. 129–132, the relaxation dynamics of the nascent hydrated electron towards the equilibrated hydrated electron can now be followed by time-resolved photoelectron spectroscopy, which certainly will provide novel information on the structure and relaxation kinetics of the transient species in electron solvation (the incompletely solvated or “wet electrons”48,49). Polarization-resolved photoemission spectroscopy is expected to emerge soon and will have the potential to provide novel information on the electron density distribution at the interface and in the bulk. Work along these lines is presently underway in our laboratories. Clearly, theory is needed to help us in the development of the complete picture of the structure and dynamics of the hydrated electron.
References
- E. J. Hart and M. Anbar, The Hydrated Electron, Wiley, New York, 1970 Search PubMed.
- E. J. Hart and J. W. Boag, J. Am. Chem. Soc., 1962, 84, 4090 CrossRef CAS.
- F. Y. Chou and G. R. Freeman, J. Phys.Chem., 1977, 81, 109 CrossRef.
- G. V. Buxton, C. L. Greenstock, W. P. Helman and A. B. Ross, J. Phys. Chem. Ref. Data, 1988, 17, 513 CAS.
- B. C. Garrett, et al. , Chem. Rev., 2005, 105, 355 CrossRef CAS.
- C. von Sonntag, Free-Radical-Induced DNA Damage and its Repair, Springer, Heidelberg, 2006 Search PubMed.
- G. Stein, Discuss. Faraday Soc., 1952, 12, 227 RSC.
- R. L. Platzmann, Radiat. Res., 1955, 2, 1 CrossRef.
- J. J. Weiss, Annu. Rev. Phys. Chem., 1953, 4, 143 CrossRef CAS.
- J. Jortner, J. Chem. Phys., 1959, 30, 839 CrossRef CAS.
- M. Natori and T. J. Watanabe, J. Phys. Soc. Jpn., 1966, 21, 1573 CrossRef CAS.
- S. Schlick, P. A. Narayama and L. Kevan, J. Chem. Phys., 1976, 64, 3153 CrossRef CAS.
- D.-F. Feng and L. Kevan, Chem. Rev., 1980, 80, 1 CrossRef CAS.
- P. J. Rossky and J. Schnitker, J. Phys. Chem., 1988, 92, 4277 CrossRef CAS.
- A. Staib and D. Borgis, J. Chem. Phys., 1995, 103, 2642 CrossRef CAS.
- B. J. Schwartz and P. J. Rossky, J. Chem. Phys., 1994, 101, 6902 CrossRef.
- L. Turi and D. Borgis, J. Chem. Phys., 2002, 117, 6186 CrossRef CAS.
- L. Turi, G. Hantal, P. J. Rossky and D. Borgis, J. Chem. Phys., 2009, 131, 024119 CrossRef.
- L. D. Jacobsen and J. M. Herbert, J. Am. Chem. Soc., 2010, 132, 10000 CrossRef.
- R. E. Larsen, W. J. Glover and B. J. Schwartz, Science, 2010, 329, 65 CrossRef CAS.
- K. D. Jordan and M. A. Johnson, Science, 2010, 329, 42 CrossRef CAS.
- H. F. Hameka, G. W. Robinson and C. J. Marsden, J. Phys. Chem., 1987, 91, 3150 CrossRef CAS.
- T. R. Tuttle Jr. and S. Golden, J. Phys. Chem., 1991, 95, 5725 CrossRef.
- F. F. Muguet and G. W. Robinson, in Ultrafast Reaction Dynamics and Solvent Effects, ed. Y. Gauduel and P. J. Rossky, AIP Conference Series, 1994, p. 158 Search PubMed.
- A. L. Sobolewski and W. Domcke, Phys. Chem. Chem. Phys., 2002, 4, 4 RSC.
- A. L. Sobolewski and W. Domcke, J. Chem. Phys., 2005, 122, 184320 CrossRef.
- A. L. Sobolewski and W. Domcke, Phys. Chem. Chem. Phys., 2007, 9, 3818 RSC.
- F. Uhlig, O. Marsalek and P. Jungwirth, Phys. Chem. Chem. Phys., 2011 DOI:1039/c1cp20764d.
- M. U. Sander, K. Luther and J. Troe, J. Phys. Chem., 1993, 97, 11489 CrossRef CAS.
- R. A. Crowell and D. M. Bartels, J. Phys. Chem., 1996, 100, 17940 CrossRef CAS.
- C. L. Thomsen, D. Madsen, S. R. Keiding, J. Thøgersen and O. Christiansen, J. Chem. Phys., 1999, 110, 3453 CrossRef.
- A. Hertwig, H. Hippler and A. N. Unterreiner, Phys. Chem. Chem. Phys., 1999, 1, 5633 RSC.
- M. Assel, R. Laenen and A. Laubereau, Chem. Phys. Lett., 2000, 317, 13 CrossRef CAS.
- S. Pommeret, F. Gobert, M. Mostafavi, I. Lampre and J.-C. Mialocq, J. Phys. Chem. A, 2001, 105, 11400 CrossRef CAS.
- C. G. Elles, A. E. Jailaubekov, R. A. Cromwell and S. E. Bradforth, J. Chem. Phys., 2006, 125, 044515 CrossRef.
- S. Pommeret, R. Naskrecki, P. van der Meulen, M. Menard, G. Vigneron and T. Gustavsson, Chem. Phys. Lett., 1988, 288, 833 CrossRef.
- J. A. Kloepfer, V. H. Vilchiz, V. A. Lenchenkov and S. E. Bradforth, Chem. Phys. Lett., 1988, 298, 120 CrossRef.
- V. H. Vilchiz, J. A. Kloepfer, A. M. Germaine, V. A. Lenchenkov and S. E. Bradforth, J. Phys. Chem. A, 2001, 105, 1711 CrossRef CAS.
- M. C. Sauer Jr., R. A. Crowell and I. A. Shkrob, J. Phys. Chem. A, 2004, 108, 5490 CrossRef.
- X. Chen and S. E. Bradforth, Annu. Rev. Phys. Chem., 2008, 59, 203 CrossRef CAS.
- L. I. Grossweiner, G. W. Swenson and E. F. Zwicker, Science, 1963, 141, 805 CAS.
- J. Feitelson, Photochem. Photobiol., 1971, 13, 87 CrossRef CAS.
- D. V. Bent and E. Hayon, J. Am. Chem. Soc., 1975, 97, 2599 CrossRef CAS.
- J. C. Mialocq, E. Amouyal, A. Bernas and D. Grant, J. Phys. Chem., 1982, 86, 3173 CrossRef CAS.
- Y. Hirata, N. Murata, Y. Tonioka and N. Mataga, J. Phys. Chem., 1989, 93, 4257 Search PubMed.
- J. Peon, G. C. Hess, J.-M. L. Pecourt, T. Yuzawa and B. Kohler, J. Phys. Chem. A, 1999, 103, 2460 CrossRef CAS.
- J. A. Kloepfer, V. H. Vilchiz, V. A. Lenchenkov, A. M. Germaine and S. E. Bradforth, J. Chem. Phys., 2000, 113, 6288 CrossRef CAS.
- F. H. Long, H. Lu and K. B. Eisenthal, Phys. Rev. Lett., 1990, 64, 1469 CrossRef CAS.
- M. Assel, R. Laenen and A. Laubereau, J. Chem. Phys., 1999, 111, 6869 CrossRef CAS.
- A. Thaller, R. Laenen and A. Laubereau, Chem. Phys. Lett., 2004, 398, 459 CrossRef CAS.
- D. M. Bartels, K. Takahashi, J. A. Cline, T. W. Marin and C. D. Jonah, J. Phys. Chem. A, 2005, 109, 1299 CrossRef CAS.
- R. Lian, R. A. Crowell and I. A. Shkrob, J. Phys. Chem. A, 2005, 109, 1510 CrossRef CAS.
- C.-R. Wang, T. Luo and Q.-B. Lu, Phys. Chem. Chem. Phys., 2008, 10, 4463 RSC.
- Y. Kimura, J. C. Alfano, P. K. Walhout and P. F. Barbara, J. Phys. Chem., 1994, 98, 3450 CrossRef CAS.
- K. Yokoyama, C. Silva, D. H. Son, P. K. Walhout and P. F. Barbara, J. Phys. Chem. A, 1998, 102, 6957 CrossRef CAS.
- M. F. Emde, A. Baltuska, A. Kummrow, M. S. Pshenichnikov and D. A. Wiersma, Phys. Rev. Lett., 1998, 80, 4645 CrossRef CAS.
- A. Baltuska, M. F. Emde, M. S. Pshenichnikov and D. A. Wiersma, J. Phys. Chem. A, 1999, 103, 10065 CrossRef CAS.
- K. F. Wong and P. J. Rossky, J. Phys. Chem. A, 2001, 105, 2546 CrossRef CAS.
- R. E. Larsen, M. J. Bedard-Hearn and B. J. Schwartz, J. Phys. Chem. B, 2006, 110, 20055 CrossRef CAS.
- D. Borgis, P. J. Rossky and L. Turi, J. Chem. Phys., 2006, 125, 064501 CrossRef.
- D. Borgis, P. J. Rossky and L. Turi, J. Chem. Phys., 2007, 127, 174508 CrossRef.
- M. Armbruster, H. Haberland and H. G. Schindler, Phys. Rev. Lett., 1981, 47, 323 CrossRef CAS.
- H. Haberland, H. Langosch, H. G. Schindler and D. R. Worsnop, J. Phys. Chem., 1984, 88, 3903 CrossRef CAS.
- G. H. Lee, S. T. Arnold, J. G. Eaton, H. W. Sarkas, K. H. Bowen, C. Ludewigt and H. Haberland, Z. Phys. D: At., Mol. Clusters, 1991, 20, 9 CrossRef CAS.
- J. V. Coe, G. H. Lee, J. G. Eaton, S. T. Arnold, H. W. Sarkas, K. H. Bowen, C. Ludewigt, H. Haberland and D. R. Worsnop, J. Chem. Phys., 1990, 92, 3980 CrossRef CAS.
- J. Kim, I. Becker, O. Cheshnovsky and M. A. Johnson, Chem. Phys. Lett., 1998, 297, 90 CrossRef CAS.
- G. Markov and A. Nitzan, J. Phys. Chem. A, 1994, 98, 3459 Search PubMed.
- J. Jortner, Z. Phys. D: At., Mol. Clusters, 1992, 24, 247 CrossRef CAS.
- J. V. Coe, S. T. Arnold, J. G. Eaton, G. H. Lee and K. H. Bowen, J. Chem. Phys., 2006, 125, 014315 CrossRef.
- J. V. Coe, S. M. Williams and K. H. Bowen, Int. Rev. Phys. Chem., 2008, 27, 27 CrossRef CAS.
- R. N. Barnett, U. Landman, C. L. Cleveland and J. Jortner, J. Chem. Phys., 1988, 88, 4429 CrossRef CAS.
- R. N. Barnett, U. Landman, C. L. Cleveland and J. Jortner, J. Chem. Phys., 1988, 88, 4421 CrossRef CAS.
- R. N. Barnett, U. Landman, G. Makov and A. Nitzan, J. Chem. Phys., 1990, 93, 6226 CrossRef CAS.
- J. R. R. Verlet, A. E. Bragg, A. Kammrath, O. Cheshnovsky and D. M. Neumark, Science, 2005, 307, 93 CrossRef CAS.
- D. H. Paik, I. R. Lee, D. S. Yang, J. S. Baskin and A. H. Zewail, Science, 2004, 306, 672 CrossRef CAS.
- A. E. Bragg, J. R. R. Verlet, A. Kammrath, O. Cheshnovsky and D. M. Neumark, Science, 2004, 306, 669 CrossRef CAS.
- N. I. Hammer, J. R. Roscioli, J. C. Bopp, J. M. Headrick and M. A. Johnson, J. Chem. Phys., 2005, 123, 244311 CrossRef.
- J. R. Roscioli, N. I. Hammer and M. A. Johnson, J. Phys. Chem. A, 2006, 110, 7517 CrossRef CAS.
- D. M. Neumark, Mol. Phys., 2008, 106, 2183 CrossRef CAS.
- A. Kammrath, J. R. R. Verlet, G. B. Griffin and D. M. Neumark, J. Chem. Phys., 2006, 125, 076101 CrossRef.
- L. Ma, K. Majer, F. Chirot and B. von Issendorff, J. Chem. Phys., 2009, 134, 144303 CrossRef.
- O. Marsalek, F. Uhlig, T. Frigato, B. Schmidt and P. Jungwirth, Phys. Rev. Lett., 2010, 105, 043002 CrossRef.
- O. Marsalek, F. Uhlig and P. Jungwirth, J. Phys. Chem. C, 2010, 114, 20489 CAS.
- O. Marsalek, F. Uhlig, J. vande Vondele and P. Jungwirth, Annu. Rev. Phys. Chem., 2011 Search PubMed , in press.
- L. Turi, W. S. Sheu and P. J. Rossky, Science, 2005, 309, 914 CrossRef CAS.
- P. Ayotte and M. A. Johnson, J. Chem. Phys., 1997, 106, 811 CrossRef CAS.
- N. I. Hammer, J. W. Shin, J. M. Headrick, E. G. Diken, J. R. Roscioli, G. H. Weddle and M. A. Johnson, Science, 2004, 306, 675 CrossRef CAS.
- T. Frigato, J. vande Vondele, B. Schmidt, C. Schütte and P. Jungwirth, J. Phys. Chem. A, 2008, 112, 6125 CrossRef CAS.
- L. Turi, W. S. Sheu and P. J. Rossky, J. Chem. Phys., 2006, 125, 014308 CrossRef.
- A. Madarasz, P. J. Rossky and L. Turi, J. Chem. Phys., 2009, 130, 124319 CrossRef.
- A. Madarasz, P. J. Rossky and L. Turi, J. Phys. Chem. A, 2010, 114, 2331 CrossRef CAS.
- A. Khan, J. Chem. Phys., 2006, 125, 024307 CrossRef.
- H. M. Lee and K. S. Kim, J. Chem. Phys., 2002, 117, 706 CrossRef CAS.
- A. L. Sobolewski and W. Domcke, J. Phys. Chem. A, 2003, 107, 1557 CrossRef CAS.
- T. Sommerfeld, S. D. Gardner, A. DeFusco and K. D. Jordan, J. Chem. Phys., 2007, 125, 174301 CrossRef.
- J. M. Herbert and M. Head-Gordon, J. Phys. Chem. A, 2005, 109, 5217 CrossRef CAS.
- W. Weyl, Ann. Phys. (Leipzig), 1864, 121, 601 Search PubMed.
- R. Takasu, F. Misaizu, K. Hashimoto and K. Fuke, J. Phys. Chem. A, 1997, 103, 3078 CrossRef.
- I. V. Hertel, C. Hüglin, C. Nitsch and C. P. Schulz, Phys. Rev. Lett., 1991, 67, 1767 CrossRef CAS.
- C. P. Schulz, R. Haugstätter, H.-U. Tittes and I. V. Hertel, Z. Phys. D: At., Mol. Clusters, 1988, 10, 279 CrossRef CAS.
- C. P. Schulz, C. Bobbert, N. M. T. Shimasoto, K. Daigoku and K. Hashimoto, J. Chem. Phys., 2003, 119, 11620 CrossRef CAS.
- C. P. Schulz and C. Nitsch, J. Chem. Phys., 1997, 107, 9794 CrossRef CAS.
- H. T. Liu, J. P. Müller, N. Zhavoronkov, C. P. Schulz and I. V. Hertel, J. Phys. Chem. A, 2010, 114, 1508 CrossRef CAS.
- C. Steinbach, M. Farnik, I. Ettischer, J. Siebers and U. Buck, Phys. Chem. Chem. Phys., 2006, 8, 2752 RSC.
- U. Buck, I. Dauster, B. Gao and Z. F. Liu, J. Phys. Chem. A, 2007, 111, 12355 CrossRef CAS.
- F. Misaizu, K. Tsukamoto, M. Sanekata and K. Fuke, Chem. Phys. Lett., 1992, 188, 241 CrossRef CAS.
- R. N. Barnett and U. Landman, Phys. Rev. Lett., 1993, 70, 1775 CrossRef CAS.
- T. Tsurusawa and S. Iwata, J. Phys. Chem. A, 1999, 103, 6134 CrossRef CAS.
- K. Hashimoto and K. Morokuma, J. Am. Chem. Soc., 1994, 116, 11436 CrossRef CAS.
- B. Gao and Z. F. Liu, J. Chem. Phys., 2007, 126, 084501 CrossRef.
- R. M. Forck, I. Dauster, Y. Schieweck, T. Zeuch, U. Buck, M. Ončák and P. Slavíček, J. Chem. Phys., 2010, 132, 221102 CrossRef.
- A. L. Sobolewski and W. Domcke, J. Phys. Chem. A, 2002, 106, 4158 CrossRef CAS.
- F. Y. Chou and G. R. Freeman, J. Phys. Chem., 1979, 83, 5570 Search PubMed.
- D. M. Hudgins and R. F. Porter, Int. J. Mass Spectrom. Ion Phys., 1994, 130, 49 CrossRef CAS.
- G. I. Gellene and R. F. Porter, J. Chem. Phys., 1984, 81, 5570 CrossRef CAS.
- M. Ončák, P. Slavíček, V. Poterya, M. Fárník and U. Buck, J. Phys. Chem. A, 2008, 112, 5344 CrossRef.
- V. Poterya, M. Fárník, P. Slavíček, U. Buck and V. V. Kresin, J. Chem. Phys., 2007, 126, 071101 CrossRef.
- M. Ončák, P. Slavíček, M. Fárník and U. Buck, J. Phys. Chem. A, 2011, 115, 6155 CrossRef.
- U. Buck, J. Phys. Chem. A, 2002, 106, 10049 CrossRef CAS.
- M. Fárník, N. H. Nahler, U. Buck, P. Slavíček and P. Jungwirth, Chem. Phys., 2005, 315, 161 CrossRef.
- P. Slavíček, P. Jungwirth, M. Lewerenz, N. H. Nahler, M. Fárník and U. Buck, J. Chem. Phys., 2004, 120, 4498 CrossRef.
- V. Poterya, J. Fedor, A. Pysanenko, O. Tkákč, J. Lengyel, M. Ončák, P. Slavíček and M. Fárník, Phys. Chem. Chem. Phys., 2011, 13, 2250 RSC.
- A. L. Sobolewski and W. Domcke, Phys. Chem. Chem. Phys., 2005, 7, 970 RSC.
- J. P. Devlin, N. Uras, J. Sadlej and V. Buch, Nature, 2002, 417, 269 CrossRef CAS.
- H. Kang, T. H. Shin, S. C. Park, I. K. Kim and S. J. Han, J. Am. Chem. Soc., 2000, 122, 9842 CrossRef CAS.
- J. Brudermann, U. Buck and V. Buch, J. Phys. Chem., 2002, 106, 453 CrossRef CAS.
- D. P. Hydutsky, N. J. Bianco and J. A. W. Castleman, Chem. Phys. Lett., 2009, 476, 15 CrossRef CAS.
- T. E. Dermota, D. P. Hydutsky, N. J. Bianco and A. W. Castleman, J. Chem. Phys., 2005, 123, 214308 CrossRef CAS.
- K. R. Siefermann, Y. Liu, E. Lugovoy, O. Link, M. Faubel, U. Buck, B. Winter and B. Abel, Nat. Chem., 2010, 2, 274 CrossRef CAS.
- Y. Tang, H. Shen, K. Sekiguchi, N. Kurahashi, T. Mizumo, Y. Suzuki and T. Suzuki, Phys. Chem. Chem. Phys., 2010, 12, 3653 RSC.
- A. T. Shreve, T. A. Yen and D. M. Neumark, Chem. Phys. Lett., 2010, 493, 216 CrossRef CAS.
- A. Lübcke, F. Buchner, N. Heine, I. V. Hertel and T. Schulz, Phys. Chem. Chem. Phys., 2010, 12, 14629 RSC.
- J. W. Chen, Y. J. Lee, H. S. Cheng and S. J. Yeh, J. Chin. Chem. Soc. (Taipei), 1974, 201, 105 Search PubMed.
- Y. Tang, Y.-I. Suzuki, H. Shen, N. Sekiguchi, N. Kurahashi, K. Nishizawa, P. Zuo and T. Suzuki, Chem. Phys. Lett., 2010, 494, 111 CrossRef CAS.
- O. Link, E. Lugovoy, K. Siefermann, Y. Liu, M. Faubel and B. Abel, Appl. Phys. A: Mater. Sci. Process., 2009, 96, 117 CrossRef CAS.
- O. Link, E. Vöhringer-Martinez, E. Lugovoj, Y. Liu, K. Siefermann, M. Faubel, H. Grubmüller, R. B. Gerber, Y. Miller and B. Abel, Faraday Discuss., 2009, 141, 67 RSC.
- P. B. Peterson and R. J. Saykally, J. Am. Chem. Soc., 2005, 127, 15446 CrossRef.
- D. Emfietzoglou, I. Kyriakou, I. Abril, R. Garcia-Molina, I. D. Petsalakis, H. Nikjoo and A. Pathak, Nucl. Instrum. Methods Phys. Res., Sect. B, 2009, 267, 45 CrossRef CAS.
- N. Ottosson, M. Faubel, S. E. Bradforth, P. Jungwirth and B. Winter, J. Electron Spectrosc. Relat. Phenom., 2010, 177, 60 CrossRef CAS.
- D. M. Sagar, C. D. Bain and J. R. R. Verlet, J. Am. Chem. Soc., 2010, 132, 6917 CrossRef CAS.
- M. Shoji, K. Kaniwa, Y. Hiranuma, O. Maselli and F. Mafune, J. Phys. Chem. A, 2011, 115, 2148–2154 CrossRef CAS.
- A. L. Sobolewski and W. Domcke, unpublished, 2011.
- R. N. Barnett and U. Landman, unpublished, 2011.
- R. N. Barnett, R. Gininger, O. Cheshnovsky and U. Landman, J. Phys. Chem. A, 2011, 115, 7378 CrossRef CAS.
- C. Steinbach and U. Buck, J. Chem. Phys., 2005, 122, 134301 CrossRef CAS.
- J. Häsing, Ann. Phys. (Leipzig), 1940, 37, 509 Search PubMed.
- H. Aulich, B. Baron, P. Delahay and R. Lugo, J. Chem. Phys., 1973, 58, 4439 CrossRef CAS.
- H. Shen, N. Kurahashi, T. Horio, K. Sekiguchi and T. Suzuki, Chem. Lett., 2010, 39, 668 CrossRef CAS.
- I. Dauster, M. A. Suhm, U. Buck and T. Zeuch, Phys. Chem. Chem. Phys., 2008, 10, 83 RSC.
- A. Kammrath, J. R. R. Verlet, G. B. Griffin and D. M. Neumark, J. Chem. Phys., 2006, 125, 171102 CrossRef.
| This journal is © the Owner Societies 2012 |