Modes of reasoning in self-initiated study groups in chemistry
Karen
Christian
a and
Vicente
Talanquer
*b
aDepartment of Chemistry and Biochemistry, University of Arizona, Tucson, Arizona 85721, USA. E-mail: christik@email.arizona.edu
bDepartment of Chemistry and Biochemistry, University of Arizona, Tucson, Arizona 85721, USA. E-mail: vicente@u.arizona.edu
First published on 11th April 2012
Abstract
Characterizing the modes of reasoning typically applied by students to solve different types of chemistry problems is of central importance for the design of instructional strategies that can better support their learning of specific content. Thus, the central goal of this study was to identify dominant modes of reasoning expressed by college chemistry students while working in self-initiated study groups. We were particularly interested in exploring potential relationships between modes of reasoning, content focus of group discussions, and levels of cognitive processing. Our study was based on the analysis of student conversations in 34 study sessions of 14 study groups involving over 100 students enrolled in the first semester of college organic chemistry. Our analysis indicated that most of the content-related conversations in the study could be categorized as involving at least one of four major reasoning modes: Model-based reasoning, case-based reasoning, rule-based reasoning, and symbol-based reasoning. In general, group talk was largely focused on issues of representation and structure which preferentially invoked rule-based reasoning; discussions about chemical reactions heavily relied on case-based reasoning. Overall, model-based reasoning was minimally applied. In general, over 70% of the content-related conversations corresponded to lower levels of cognitive processing. Rule-based thinking and case-based thinking were often based on students remembering and applying knowledge. Although model-based reasoning was used scarcely, it more frequently led to higher levels of cognitive processing. The results of our work provide insights into how to modify instruction to better support students' reasoning inside and outside the classroom.
Introduction
College chemistry instructors often are aware that a significant fraction of their students participate in self-initiated student groups formed outside the classroom for studying purposes. However, little is actually known about the structure and dynamics of these types of groups or about the approaches to studying followed by group participants (Tang, 1993). Although study strategies have been investigated by various authors (Entwistle, 2000; Flippo and Caverly, 2008), most of the information that is available is largely based on individual student self-reports (Winne and Jamieson-Noel, 2002). Considering that collaborative work in independent study groups can be expected to be self-selected, self-structured, and self-regulated by the participating students, direct observation and analysis of these types of groups could help us identify potential strategies to support student learning inside and outside the classroom.To develop a better understanding of the approaches to studying and learning of students working in self-initiated study groups, we observed and analyzed the work of science and engineering majors enrolled in the first semester of organic chemistry at the college level. We were particularly interested in exploring potential relationships between modes of reasoning, content focus of group discussions, and levels of cognitive processing. From this perspective, our study contributes to our understanding of problem-solving approaches used by college students working in collaborative groups. A large proportion of studies on problem-solving in chemistry have been focused on the analysis of general strategies applied by students to solve quantitative problems (Gabel and Bunce, 1994; Bodner and Herron, 2002). Much less is known about the domain-specific ways of thinking on which students rely to solve qualitative problems such as those characteristic of organic chemistry. Although some researchers have explored reasoning strategies used by individual undergraduate and graduate organic chemistry students while engaged in interview tasks (Bhattacharyya and Bodner, 2005; Anderson and Bodner, 2008; Ferguson and Bodner, 2008; Cartrette and Bodner, 2010; Kraft et al., 2010), our results provide insights into what college chemistry students actually do when reviewing course content on their own.
Modes of reasoning in chemistry
Investigations of student reasoning while answering chemistry questions or solving chemistry problems have followed a variety of approaches. Many of these studies have focused on analyzing how students' prior knowledge affect both their comprehension of core chemistry concepts and the development of mental models to make predictions and build explanations in a variety of areas, from atomic structure to chemical bonding to chemical equilibrium (Gilbert et al., 2002; Taber 2002; Kind, 2004). Results from these types of research projects suggest that chemistry students at all educational levels can be expected to express a variety of alternative conceptions as a result of the application of a set of more or less integrated cognitive resources that guide but also constrain their reasoning (Talanquer, 2006; Taber and García-Franco, 2010).A variety of researchers have also analyzed students' problem-solving strategies when dealing with prototypical academic tasks (Gabel and Bunce, 1994; Bodner and Herron, 2002). Many of these studies have focused on students working on quantitative problems that can be solved mathematically. Results from these investigations indicate that students tend to strongly rely on means-ends analysis strategies (Heyworth, 1999), that their success in problem solving is not necessarily coupled to deep conceptual understanding (Nurrenbern and Pickering, 1987), and that problem solving is often accomplished with heavy reliance on the application of algorithms (Anderson and Bodner, 2008). The overuse of both domain-general and domain-specific short-cut heuristics to solve qualitative chemistry problems, such as classification (Stains and Talanquer, 2007, 2008), completing word equations (Taber and Bricheno, 2009), and ranking substances (Maeyer and Talanquer, 2010; McClary and Talanquer, 2011) has also been highlighted by some authors.
Recently, Bhattacharyya and coworkers (Kraft et al., 2010) have shown that the reasoning processes used by graduate students to solve organic chemistry problems may be quite varied and may evolve as students work on a given task. In particular, these authors identified three major modes of reasoning used by their study participants labeled as model-based reasoning (MBR), case-based reasoning (CBR), and rule-based reasoning (RBR). These reasoning approaches to solving qualitative chemistry problems can be thought of as instances of domain-specific cognition that result from domain-general reasoning processes operating on domain-specific representations. In this sense, to clearly characterize each of them we need to analyze both the general cognitive processes on which they are based as well as the particularities of their instantiation in the domain of chemistry.
Model-based reasoning is commonly used and highly valued in the sciences, particularly in chemistry where a wide diversity of models have been developed to describe, explain, and predict the structure and properties of matter (Erduran and Duschl, 2004; Justi and Gilbert, 2006). These models represent chemical systems by attributing to them some sort of internal structure, composition, or mechanism that serve the purpose of explaining or predicting the various properties or behaviors that are observed. Chemistry is, to a large extent, a science of abstractions in which models are developed and used to understand matter at different scales (e.g., macroscopic, sub-microscopic), in various dimensions (e.g., composition/structure, energy, time), and following different approaches (e.g., conceptual, mathematical) (Talanquer, 2011). These models can be static or dynamic; they may be represented in concrete, visual, or verbal modes. Ultimately, they provide many of the tools we use to build and communicate chemical knowledge. Results from research in science education indicate that students are able to apply model-based reasoning when engaged in problem-solving, but the models they express tend to be hybrid entities that combine scientific ideas with common-sense beliefs about the properties and behavior of the systems of interest (Vosniadou, 1994).
In certain problem solving situations, humans also use old experiences (cases) to solve the problems that they are facing (Kolodner, 1993). This type of case-based reasoning may imply adapting old solutions to meet new demands, using old cases to interpret or explain new situations, or using past experiences to create or critique solutions to a new problem. By referring to known cases, individuals are able to use information that is familiar to them in order to solve a less familiar task. In this way, instances of similarity between a problem and a previous case provide the necessary overlap to reason through a novel situation. Rather than trying to piece together many pieces of information, a person can solve a problem as a complete unit by referring to an analogous case. Case-based reasoning is dependent on two main aspects: the knowledge of past experiences and the ability to remember and apply these experiences appropriately to a new situation (Leake, 1996).
Case-based reasoning plays a central role in chemical thinking (Kovac, 2002). A significant portion of our chemistry knowledge is organized in sets of interconnected classification systems that condense and systematize a vast amount of information about the properties of chemical substances and processes. Classification in chemistry is a powerful tool for prediction as it allows us to infer the potential behavior of a chemical substance based on the analysis of its composition and structure (Schummer, 1998). Classification is also an invaluable instrument for decision-making as it supports a sort of “casuistry of chemistry,” in which known types of chemical substances or reactions serve as paradigmatic cases that help chemists make effective decisions about potential synthetic routes or methods of analysis (Kovac, 2002). The power of case-based reasoning in chemistry has been recognized in the development of computational systems for predicting reactions (Elrod Maggiora and Trenary, 1990) or proposing synthetic pathways (Gelernter et al., 1990). Unfortunately, little is known about how students apply this type of reasoning to solve chemistry problems.
People also have the ability to induce patterns of behavior from direct experience with the world or from the mental models depicted in their minds. These resulting patterns of behavior can then be cast into empirical generalizations or symbolic rules to simplify thinking about future problems or to reveal higher order relationships (Schwartz and Black, 1996). These “rules” can serve as heuristics to more easily draw inferences and make quick predictions and decisions. This type of rule-based reasoning can be quite efficient in generating satisfactory answers with low cognitive effort and processing time. Unfortunately, it can also lead to systematic errors and reasoning biases when applied indiscriminately across many situations (Gilovich et al., 2002). Novices often fail to recognize the proper cues that justify the use of a given rule and tend to overextend the scope of its application.
A significant fraction of our theoretical and practical knowledge in chemistry is often represented in the form of rules (Kovac, 2002). For example, there are a variety of normative rules to name, represent, and classify chemical substances and processes. There are rules that capture general patterns in properties and behavior, like those that describe periodic trends for elemental atoms across the periodic table. There are empirical rules about the properties of chemical compounds, such as “like dissolve like.” There are theoretical rules that look to provide general explanations to observed patterns, such as the “octet rule” and “Le Chatelier's Principle.” These different types of rules are extremely useful in the analysis and prediction of chemical structure and reactivity. However, most of them have restricted ranges of application and often involve exceptions. Results from research in chemical education indicate that chemistry students tend to overlook these limitations and over-rely on rule-based reasoning in making predictions and building explanations (Gabel and Bunce, 1994; Anderson and Bodner, 2008; Stains and Talanquer, 2007, 2008).
Levels of cognitive processing
Student reasoning in chemistry has also been analyzed from the perspective of the level of cognitive processing that is involved. However, the discussion has been framed in different ways by different authors. For example, several studies in chemical education have focused on the algorithmic versus conceptual nature of traditional academic chemistry problems and questions, as well as on the levels of reasoning that each category of questions are likely to trigger (Nurrenbern and Pickering, 1987; Gabel and Bunce, 1994). Other authors have considered the extent to which various instructional and assessment strategies promote the use and development of lower-order cognitive skills (LOCS) versus higher-order cognitive skills (HOCS) (Zoller and Tsaparlis, 1997). Recently, it has been suggested (Smith et al., 2010) that the so-called revised Bloom's taxonomy (Anderson and Krathwohl, 2001; Krathwohl, 2002) provides a useful framework for conceptualizing and organizing existing work in this area.The revised Bloom's taxonomy describes six different levels of cognitive processing identified as remember, understand, apply, analyze, evaluate, and create. The taxonomy is hierarchical in the sense that the six major categories are believed to differ in complexity, with remember being less complex than understand, which is seen as less complex than apply, and so on. Although the categories can be expected to overlap one another to some extent, empirical evidence suggests that the “center point” define a scale from simple to complex cognitive processing (Anderson and Krathwohl, 2001).
Research goals
The central goals of this study were to investigate the modes of reasoning, content focus, and levels of cognitive processing that characterized students' content-related conversations in self-initiated study groups. In particular, our investigation was guided by the following research questions:• What major modes of reasoning do students apply while reviewing organic chemistry content in self-initiated study groups?
• How does the content focus of group discussions affect reasoning approaches?
• What levels of cognitive processing are most frequently associated with the different modes of reasoning observed in the study groups?
Methodology
Context and participants
This study was conducted in a public university in the southwestern United States. Study participants were science and engineering majors enrolled in different sections of the first organic chemistry lecture course offered at this institution during three consecutive academic semesters. Participants were recruited in organic chemistry lectures and labs by asking for volunteers who were planning to form study groups in preparation for the course and were willing to have an observer in their meetings. From this perspective, we did not have any control on the size, composition, or frequency of meetings of any of the observed groups; our goal was to observe as many groups as possible under the conditions in which they normally worked. Some of these groups met regularly throughout the academic semester, and others ended up meeting only once or twice.A total of 131 individuals (69 females and 62 males) organized in 14 dynamic self-initiated study groups participated in the study. We highlight the word ‘dynamic’ to emphasize the variability in size, composition, and meeting frequency of the observed self-initiated groups. Analysis of general achievement data indicated that, on average, participants in our study had higher grade point averages (GPA = 2.78, in a scale of 0 to 4) than the average for all of the students in the organic chemistry course (GPA = 2.20).
Data collection
Research data included observation notes and full transcripts of audio recordings of 34 different study sessions. In each case, general observations were made to register the meeting location, the types of resources used by the students (such as textbook or class notes), and other details of the group composition (e.g., size, gender of participants). During most study sessions students drew molecular structures and other chemical symbols on a whiteboard or in their notes. Copies of these representations were made to the extent that was possible without disturbing the group dynamics; these drawings were later embedded into the appropriate points of a session's transcript to facilitate their analysis and interpretation.Study group meetings took place in a variety of locations, often in the library, in study rooms, or in students' dormitory lounges. Meetings could last for several hours but observations were limited to an average length of 60 min per study session. In all of the cases, group participants made their own decisions about when and where to meet and what and how to study. The first author of this work was informed of the meetings and invited to attend the sessions via e-mail. During a study session, this researcher remained as a detached observer of the group, refraining from initiating any content-related conversation with the participants. Observer initiated interactions were limited to ensuring informed consent of all group participants, and to informal introductions and conversations that helped build rapport with the students.
Study sessions were observed as many times as possible. We made every effort to complete observations throughout the different academic semesters, and encouraged each study group to invite us back to observe their work multiple times. However, given our lack of control on meeting schedules and students' decisions to invite us to a study session, not all of the study groups were observed the same number of times. Thus, of the 14 study groups that participated in the study, seven of them were only observed once, three of them were observed in two different occasions, two groups were visited three times, one group was observed five times, and one group was visited in 10 occasions. It is important to point out that a significant proportion of the self-initiated study groups observed in our study were highly dynamic in their size and composition. This variability is likely an intrinsic feature of self-initiated study groups in organic chemistry. Students' needs, interests, and availability can be expected to vary across a semester, and people may join or leave a study group as the semester progresses. The goal of our study was to analyze the nature of students' interactions in these realistic settings and not under artificial designed conditions (e.g., by creating or looking for fixed-composition study groups).
Observations of study groups were made throughout the academic semester as we tried to explore how students studied at different points in their classes. Generally, students met in preparation for one of the four midterm exams or for the comprehensive final exam in the organic chemistry course. When students met to prepare for the final exam, it was common for them to organize separate study sessions targeting the content of a specific earlier midterm exam. For purposes of analysis, such study sessions were grouped with the sessions corresponding to that same exam.
Data analysis
Audio recordings from each of the study group sessions were transcribed and analyzed in order to identify instances where students used different modes of reasoning. The transcripts were first examined looking for episodes in which students were on-topic and discussing chemistry content. Segments where students were off-task were not analyzed. Results from prior studies on problem-solving in chemistry (Kovac, 2002; Kraft et al., 2010; Raker and Towns, 2010) were used to define a first set of coding categories for the analysis of modes of reasoning (e.g. model-based reasoning, case-based reasoning). As the analysis proceeded, additional categories were created to capture additional reasoning strategies (e.g., symbol-based reasoning). Categories to describe the content focus of group discussions emerged from direct analysis of the data. Types of categories in this area included topics such as synthesis, mechanism, and representation. A revised Bloom's taxonomy (Krathwohl, 2002) was used as a framework to identify different levels of cognitive processing exhibited by our study participants.To illustrate our analytical procedure, consider the following excerpt from a study group conversation:
O: Did you say that you understand radical mechanisms?
J: It's the same as the one in the book. Because it looks like it follows like the same pattern when they use Cl in the book?
O: That's a good example. Let's use Cl. Give me the…
J: It's the same thing exactly, except that the Br is the Cl.
In this case, students were using the known properties or behavior of chlorine radicals (a known case) to infer the behavior of bromine radicals. This episode was then coded as case-based reasoning. In contrast, the following conversation was coded as model-based reasoning because students were building a mechanistic explanation based on physical or chemical models of a system:
H: Whoah. Okay, how does that work?
A: Let me see. So that's the bromonium ion and that has a positive charge. And that's why, this has a negative charge on it? So this attacks it. So that joins there, and when the positive (something), it had to go here. And then the bromine comes in and takes the hydrogen. Does that make sense?
Those episodes coded as rule-based thinking corresponded to situations in which students applied well-known rules to make decisions or build explanations. For example:
N: Isn't identical and meso the same?
E: Are they always the same thing?
N: Well in order for it to be meso, it would have to have a plane of symmetry. And it's got to have a chiral center.
Additionally, we identified instances in which students' conversations were mostly focused on the manipulation of symbolic representations to find an answer. These types of episodes were coded as symbol-based reasoning.
Coding segments were defined at the episodic level by assigning a different code after every perceived shift in mode of reasoning. For comparison purposes, the length of an episode was determined by counting the number of words spoken during that segment. In some instances, conversations between students would involve long pauses. In other cases, students would rapidly respond to one another and overlapping conversations may ensue. Although students may have been actively thinking in these different instances, we had no reliable way of characterizing their reasoning. Thus, keeping track of the number of words rather than a span of time allowed us to elicit the comparative prevalence of different modes of reasoning or levels of cognitive processing associated with student conversations. The relative frequency of these different categories of expressed thought was determined by finding the percentage of words that corresponded to each category within any given study session. We used these frequencies to calculate average values within and across study groups. Given the high skewness of most of our data distributions, we calculated semi-interquartile ranges (SIR) instead of standard deviations (SD) as a measure of data dispersion. Due to the high asymmetry of some of our data distributions, the SIR exceeded the average value in a few cases.
Data analysis was completed in various steps. Once an initial coding system was proposed and discussed by the two researchers involved in the study, the first author of this paper applied it to the analysis of one of the transcripts. Both the proposed segmentation of the transcript and the specific codes assigned to each segment were then independently reviewed by the second researcher, who either agreed with the assignations or proposed to change them. Comparison and discussion of ideas helped refine the coding system and the identification of boundaries between segments. Once agreement was reached, the process was repeated with more transcripts until disagreements on a single transcript were less than 10% of the total segments and codes. The authors then separately coded another set of transcripts, compared and discussed their results, repeating the process until achieving over 90% agreement in consecutive sets. The resulting coding system and methodology was then applied by the first author to analyze the totality of the data.
Findings
The presentation of the results has been organized using our research questions as a guide. Thus, we first describe the modes of reasoning that were more prevalent in the observed study groups. Second, we analyze the relationship between content focus and modes of reasoning. Finally, we describe salient associations between modes of reasoning and levels of cognitive processing.Modes of reasoning
The analysis of students' talk during the different study sessions revealed four major modes of reasoning applied by students to solve chemistry problems and share or discuss course content. Three of these modes, rule-based reasoning (RBR), case-based reasoning (CBR), and model-based reasoning (MBR), correspond to those previously described by Kraft et al., (2010). The fourth mode, symbol-based reasoning (SBR), was based on the mere manipulation of chemical symbology. Observed study groups sometimes used a single mode of reasoning in approaching the solution or discussion of a chemistry problem. However, many of the groups' conversations involved more than one mode of reasoning, particularly when initial approaches to solve a problem were unsuccessful. As shown in Table 1, most of the observed study groups engaged in different modes of reasoning throughout a single study session.| Group | Session (Exam) | %SBR | %RBR | %CBR | %MBR |
|---|---|---|---|---|---|
| 1 | 1 (1) | 0.0 | 78.8 | 0.0 | 21.2 |
| 2 | 1 (1) | 0.0 | 87.3 | 0.0 | 12.7 |
| 3 | 1 (1) | 4.2 | 95.8 | 0.0 | 0.00 |
| 4 | 1 (2) | 0.0 | 96.8 | 0.0 | 3.2 |
| 5 | 1 (2) | 0.0 | 78.5 | 0.0 | 21.5 |
| 6 | 1 (3) | 0.0 | 5.8 | 86.0 | 8.3 |
| 7 | 1 (1) | 0.0 | 100.0 | 0.0 | 0.00 |
| 8 | 1 (1) | 9.9 | 63.4 | 11.8 | 14.9 |
| 2 (2) | 15.6 | 43.5 | 26.7 | 14.2 | |
| 3 (3) | 23.2 | 11.4 | 50.6 | 14.8 | |
| 4 (4) | 26.3 | 43.0 | 24.2 | 6.5 | |
| 5 (4) | 11.4 | 34.8 | 49.7 | 4.1 | |
| 9 | 1 (1) | 7.1 | 77.8 | 7.1 | 8.0 |
| 2 (1) | 0.0 | 100.0 | 0.0 | 0.0 | |
| 3 (2) | 20.6 | 48.2 | 24.0 | 7.2 | |
| 10 | 1 (1) | 5.2 | 52.0 | 42.5 | 0.0 |
| 2 (3) | 37.0 | 0.0 | 51.9 | 11.1 | |
| 11 | 1 (1) | 27.9 | 71.3 | 0.8 | 0.0 |
| 2 (1) | 7.2 | 73.2 | 19.5 | 0.0 | |
| 3 (1) | 0.4 | 82.3 | 0.0 | 17.3 | |
| 4 (2) | 2.6 | 93.0 | 4.4 | 0.0 | |
| 5 (3) | 0.0 | 78.2 | 12.6 | 9.3 | |
| 6 (4) | 2.9 | 88.0 | 4.7 | 4.5 | |
| 7 (1) | 1.7 | 98.3 | 0.0 | 0.0 | |
| 8 (3) | 0.0 | 26.3 | 73.7 | 0.0 | |
| 9 (4) | 0.0 | 20.1 | 12.7 | 67.2 | |
| 10 (Final) | 0.0 | 12.1 | 71.3 | 16.6 | |
| 12 | 1 (2) | 19.9 | 52.0 | 28.1 | 0.0 |
| 2 (4) | 14.4 | 13.2 | 69.8 | 2.7 | |
| 13 | 1 (2) | 13.1 | 76.5 | 10.4 | 0.00 |
| 2 (4) | 7.6 | 42.5 | 40.8 | 9.1 | |
| 3 (Final) | 7.1 | 19.3 | 63.1 | 10.6 | |
| 14 | 1 (2) | 12.4 | 58.6 | 26.0 | 3.1 |
| 2 (3) | 16.7 | 7.0 | 74.0 | 2.25 |
O: Now, because there's two methyls, I would say 2 comma 8 dimethyl. Neopentyl nonane. Because the “ane” goes last.
F: Yeah. And why'd you put dimethylneopentyl instead of neopentyldimethyl?
O: Because M comes before N.
F: Okay.
O: Okay. And even though there's no number it would be neopentyl dash nonane? Like, I mean because nonane is a chain, it's not like there's a location. But there's a dash.
F: No dash.
O: There's no dash.
F: Because it's dash between different things and commas between likes.
Students often cited and applied rules when solving a problem or when providing explanations to others. In these different situations, they could either apply a rule that they knew or invest time discussing how to interpret or apply the rule to the problem under analysis. Students would often apply multiple rules while working on a problem or rely on an algorithmic procedure to generate a solution.
J: What is that called? What is that reaction called?
A: Halogenation.
J: So you're going to have two halogens added on.
L: Okay. Because we're going to do, because we're doing anti, they're going to go like this. Now, this is where the double bond is, so we're going to do a Br here and a Br here.
J: Keep in mind what the intermediates look like. It's the triangle with the Br.
As illustrated by this example, the use of cases allowed students to more easily invoke structures or mechanisms associated with the substances or processes under discussion.
E: The bond between two carbons is typically equally shared. So looking at this tertiary carbocation. Typically these bonds between these carbons are the same, because they're equally shared, they're the same element, so they're indistinguishable. So, they both share the electrons perfectly, evenly. But when we put a positive charge on one of those carbons, what can happen is, is called the inductive effect. So that can pull in a little bit of the electron density that it would typically share. It says, I'm a selfish carbon, and it will keep it. This won't work with a proton, because hydrogen is just that little bit more electronegative and it won't share, so therefore you have less stabilization on the primary carbocation. So that's not stable. These guys won't help.
Students who applied this type of reasoning sometimes relied on scientifically accepted chemical models, but often expressed less developed, hybrid, or synthetic mental models during their discussions (Vosniadou, 1994).
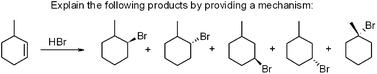 | ||
| Fig. 1 Question requiring students to propose a mechanism to explain the formation of four different products. | ||
T: It would be something like that. And then your intermediates are going to be . . . here. And then Br minus. That's what the intermediates are. That bond is going to break, it's going to go here. It will just bond like anywhere on this? That's what I think.
A: Well, this is the final product though. So the bromine doesn't leave.
T: Yeah. Like, I'm leaving that one below.
A: So just.
T: And when you have the intermediates, you don't. These two electrons will go, this bond will break, and it will go to hydrogen, and this Br will form a bond down here. So let's look and see what we have (looks at the answer) Oh. Ohh, okay. Yeah. We have to put a positive charge over here…
During these types of conversations there was no clear indication that students thought of chemical symbols as representing actual chemical substances, structures, or processes. Chemical symbols were manipulated in a semi-arbitrary fashion to solve a problem or answer a question. On average, SBR corresponded to 8.66% (SIR = 7.20) of all of the groups' talk.
Content focus
Our observations of self-initiated study groups involving college organic chemistry students indicated that a majority of the group work focused on solving problems provided (e.g., practice exams) or suggested (e.g., end-of-chapter problems in textbooks) by the course instructors. On average, this type of work corresponded to 63.6% (SIR = 32.6) of the groups' talk. In some cases students worked on self-generated problems (13.0%, SIR = 2.0) or questions (10.0%, SIR = 6.8), or used their time to review course content (13.4%, SIR = 4.8). These different types of problems induced conversations about five major content issues:These categories represent the major types of content-related conversations in which the students engaged in the observed study groups. On average, students invested close to 60% of their conversations on representational and structural issues, 20% talking about reactions, and 20% on reactivity and mechanistic issues. However, focus on each of these content areas changed over the semester. It is important to point out that the nature of the group discussions was influenced by both the study problems' statements, which in many cases framed how students' approached problem solving, but also by students' prior knowledge and interpretations. For example, if a group tried to predict a mechanistic pathway, they might have focused on the representational meaning of the arrow-pushing formalism, while other group working on a similar problem may have focused on the reactivity of the starting materials. In other cases, students may have considered, for example, issues related to representation, structure, and reactivity while trying to make sense and justify the formation of certain products in a reaction.
Table 2 displays the average percentages of group talk corresponding to different modes of reasoning as a function of the content focus of group discussions. As shown in this table, certain types of content discussions were approached using preferential types of reasoning. For example, issues of representation and structure preferentially invoked rule-based reasoning (76.6% and 54.5% of words spoken, respectively, during these types of content discussions), while conversations about “reactions” were mostly based on cased-based reasoning across different study groups (89.5% of the groups' talk on reactions). While analysis of chemical reactivity involved a high proportion of rule-based thinking (38.3%), these types of discussions were the ones most likely to trigger model-based reasoning (31.5% of group talk about reactivity). On the other hand, students' discussions about reaction mechanisms were mostly approached by applying case-based reasoning (40.1%) and symbol-based reasoning (33.7%).
| Content Focus | SBR | RBR | CBR | MBR |
|---|---|---|---|---|
| Avg% (SIR)a | Avg% (SIR) | Avg% (SIR) | Avg% (SIR) | |
| a The semi-interquartile range (SIR) was used as a measure of data dispersion. | ||||
| Representation | 15.1 (16.6) | 76.6 (15.6) | 4.9 (3.6) | 3.5 (0.0) |
| Structure | 13.0 (10.9) | 54.5 (36.2) | 19.4 (17.9) | 12.9 (9.4) |
| Reactions | 2.3 (0.0) | 8.0 (4.4) | 89.5 (7.6) | 0.2 (0.0) |
| Reactivity | 10.4 (5.1) | 38.3 24.4) | 19.9 (16.4) | 31.5 (29.5) |
| Mechanism | 33.7 (29.7) | 11.4 (9.4) | 40.1 (34.3) | 14.8 (4.5) |
Strong correlations between specific types of content discussions and particular reasoning strategies can logically be expected in some cases. For example, learning how to use chemical symbology and nomenclature to represent and name chemical substances and processes (representational issues) requires competency in applying normative rules in the discipline (rule-based reasoning). Similarly, predicting the outcome of a chemical reaction or identifying a potential synthetic pathway (reactions) is greatly simplified by classifying the process in certain category and applying case-based reasoning to infer the nature of a product or the proper steps to synthesize it. More revealing of potential deficiencies in students' approaches to studying are the observed weak correlation between model-based reasoning and discussions about structure, reactivity, and mechanism, and the major role that case-based reasoning and symbol-based reasoning had on students' discussions associated with reaction mechanisms.
In general, many of the problems related to representation and structure were approached in very similar ways by students from different study groups. Given that these types of problems were frequently approached using rule-based reasoning, this helps explain why this type of reasoning was so prevalent across groups. Similarly, students were quite consistent in the application of case-based reasoning to solve problems that required predicting reaction products or proposing synthetic pathways. A wider variability of reasoning strategies was observed in discussions about reactivity and reaction mechanisms. This variability was observed across study groups, within study groups as they worked on various problems of the same type, and even as students worked on a single problem.
Levels of cognitive processing
The analysis of students' conversations using a revised Bloom's taxonomy (Krathwohl, 2002) elicited the presence of all levels of cognitive processing:In general, over 70% of the observed content-related conversations corresponded to lower levels of cognitive processing in our coding system (Remember, Understand, and Apply). However, certain modes of reasoning were more prone to induce lower or higher levels of cognitive processing (see Table 3). For example, engagement in symbol-based reasoning and case-based reasoning was frequently based on students' recalling specific examples from their courses or remembering types of substances or processes (remembering). The use of rule-based thinking often involved “remembering” specific rules that could help solve a problem and applying them in either an algorithmic (executing) or a more interpretative (implementing) fashion. In these latter cases, a significant portion of students' discussions was spent making sense of a rule, or determining the proper rule that should be employed under a given set of circumstances. Although model-based reasoning was often associated with a single student explaining a phenomenon to help others understand it (understanding), this is the type or reasoning that more frequently led to higher levels of analytical thinking (analyzing).
| Mode of Reasoning | Remember | Understand | Apply | Analyze | Create |
|---|---|---|---|---|---|
| Avg% | Avg% | Avg% | Avg% | Avg% | |
| (SIR)a | (SIR) | (SIR) | (SIR) | (SIR) | |
| a The semi-interquartile range (SIR) was used as a measure of data dispersion. | |||||
| SBR | 52.3 | 21.4 | 13.8 | 11.7 | 0.7 |
| (39.2) | (23.8) | (10.4) | (0.0) | (0.0) | |
| RBR | 35.1 | 9.8 | 38.2 | 15.3 | 1.6 |
| (14.9) | (8.0) | (25.0) | (14.0) | (0.0) | |
| CBR | 62.6 | 12.4 | 15.0 | 9.9 | 0.0 |
| (23.6) | (7.4) | (14.7) | (8.0) | (0.0) | |
| MBR | 3.3 | 48.6 | 20.0 | 28.1 | 0.0 |
| (0.0) | (50.0) | (12.4) | (30.5) | (0.0) | |
Additional discussion
Although students;' approaches to studying have been investigated by various authors (Entwistle, 2000), most of the information that is available is largely based on individual student self-reports. In this regard, our results provide insights into what college chemistry students actually do when reviewing course content. Our study allowed us to characterize four major types of reasoning strategies used by these students to analyze and solve qualitative chemistry problems. The analysis of such reasoning processes revealed a heavy reliance on rule-based reasoning and case-based reasoning for problem-solving, with much lesser involvement in model-based reasoning during the discussion of course content or while generating solutions for study problems. Students also engaged in symbol-based reasoning, a strategy based on the manipulation of chemical symbology using a means-ends approach, particularly in the analysis and discussion of reaction mechanisms. This mode of reasoning has been identified by previous authors (Bhattacharyya and Bodner, 2005).Given the central role that solving problems had on students' study tactics, the content focus of such problems had a strong influence on student reasoning during the observed study sessions. Although the organic chemistry course addressed topics related to the characteristics of both chemical substances and processes, close to 60% of students' discussions in preparation for course exams were focused on representational and structural issues. These types of conversations triggered rule-based reasoning or case-based reasoning in close to 80% of the cases (Table 2). Discussions about the outcome of chemical reactions or synthetic pathways amounted to an average of near 20% of the groups' talk, with close to 90% of these conversations being based on cased-based reasoning. A wider variability in reasoning strategies was observed in discussions about chemical reactivity and reaction mechanisms, but these types of conversations only corresponded to a little over 20% of all of the groups' talk.
Many of the study groups invested a significant proportion of group talk on representational and structural issues rather than on the more process-oriented aspects of a problem even when working on tasks related to reactivity and reaction mechanisms. In this latter case, it was common for students to treat mechanisms as cases or structural stories to be memorized and applied in specific situations. Chemistry students' focus on the more static-structural aspects of chemical substances and reactions versus the more dynamic-process-oriented facets of chemical entities and phenomena has been reported by other authors (Anderson and Bodner, 2008; Strickland et al., 2010). The sources of this problem seem to be diverse. For example, the simultaneous use of multiple forms of representation of substances and reactions in chemistry classrooms requires students to invest considerable time and effort learning the normative rules to both create and use the representations as well as to translate between different symbolic and iconic systems. On the other hand, even if students are able to efficiently manipulate chemical representations, they may conceive them as static depictions of rigid objects that need to be assembled and disassembled to obtain the desired outcomes (Kozma and Russell, 1997).
Students' focus on the representational and structural aspects of chemistry may also be determined by the intrinsic nature of traditional chemistry curricula. Introductory chemistry courses at the secondary and college levels typically focus on the discussion of structural models and theories of matter, putting a stronger emphasis on the composition/structure dimension of chemistry than on the energy and time dimensions (Jensen, 1998). Additionally, it is common for instructors in these courses to introduce and discuss chemical representations more as visualizations of chemical entities and phenomena than as dynamic modeling tools to explore and predict the properties and behavior of the systems that they represent. The amount of information that is traditionally covered in college courses, together with the nature of conventional teaching practices at this educational level can also play a major role in students' approaches to studying. Research studies in this area indicate that the nature of the learning environment and the content and demands of the instructional and assessment tasks have a strong influence on students' approaches to learning (Entwistle, 2000).
Participants in our study were enrolled in a college course in which vast amounts of information about chemical substances and reactions were presented in a short period of time, lecturing was the prevalent teaching strategy, and assessment of learning was based on students' ability to solve traditional organic chemistry problems during classroom exams. In this context, our results suggest that the majority of the observed study groups adopted a rather strategic approach to studying (Entwistle, 2000) by using instructor-suggested problems to guide their work, focusing on the development of the knowledge and skills needed to answer old exams, and minimizing time and cognitive effort by relying on those modes of reasoning that more easily and quickly could generate an answer. Given the nature of the educational environment in which students were immersed, it may be not surprising that they relied on an instrumental approach to learning based on the implicit classification of problems into certain types and the search for and memorization of rules or cases that could be applied to solve them (Anderson and Bodner, 2008).
Students in the observed study groups were likely to make strategic study decisions based on what they perceived as the best approach to ensure success in the types of tasks and assessments in which they engaged in the classroom. They may have also had reproductive beliefs about the nature of learning (Marton and Säljö, 1997) and lacked the metacognitive knowledge needed to engage in more meaningful study practices. As a result, they may have not seen the value of engaging in model-based reasoning to analyze and solve problems or did not know how to productively do it. Their approach to learning was thus replicative (knowing that) and applicative (knowing how), focused on remembering rules and cases, trying to understand when and how to use them (Bransford and Schwartz, 1999). Comparatively, they rarely engaged in more interpretative (knowing with) ways of reasoning, analyzing and evaluating tasks in critical ways, working collaboratively to build understandings rather than just to reproduce them.
Implications
The minimal role played by model-based reasoning in the observed study groups underscores the need to critically analyze the teaching of chemistry at all educational levels. Only a few individuals in the different study groups that we observed exhibited this type of reasoning and tended to use it in a rather passive way, merely as an explanatory tool to help others understand a concept or a procedure. Using model-based reasoning at high levels of cognitive processing is cognitively taxing. Students are unlikely to engage in such type of reasoning unless they see value in doing it and the strategy is well-established in their cognitive and metacognitive skill sets. If students are to appropriate this type of thinking, teachers' instructional and assessment practices need to create opportunities for it (Justi and Gilbert, 2002; Treagust et al., 2004). Instructors need to not only model this way of reasoning in their classrooms, but also open spaces for students to actively engage in model-based reasoning for a variety of purposes (e.g., explaining, interpreting, predicting, evaluating) and in diverse contexts (e.g., structural analysis, reaction prediction, mechanistic description). They also need to modify their assessment practices to deemphasize the replicative and applicative tests of learning, and strengthen the more interpretative components (Bransford and Schwartz, 1999).Chemistry instructors should also help students understand the strengths and weaknesses of other valuable ways of thinking in the discipline. The use of rule-based thinking and case-based thinking by our study participants frequently led to low levels of cognitive processing (Table 3). However, this result is not an intrinsic characteristic of these two types of reasoning. Rules and cases are frequently applied by expert chemists in critical and thoughtful ways to solve a variety of analysis and synthesis problems (Kovac, 2002). Unfortunately, there is little research on how to foster more meaningful approaches to the use of RBR and CBR in the chemistry classroom. In this regard, analyzing related work in other fields such as engineering and medical education may be helpful. Engineering design, for example, heavily relies on the application of rules and cases to accomplish the desired goals (Eastman et al., 2001). Efficient and creative design demands rational use of rules-of-thumb and “cases of practice” based on the careful analysis of design criteria and constraints. Similarly, the application of rules and cases seems to play a central role in medical diagnosis and treatment (Kolodner, 1993). In these situations, quality of reasoning depends on people's ability to understand new situations in terms of past cases and on their capacity to both adapt past solutions and evaluate outcomes. From this perspective, student reasoning in chemistry could certainly benefit from a more explicit and reflective approach to the use of rules and cases in problem-solving in the discipline.
For the most part, the students that we observed did not spend large amounts of time applying rather unproductive ways of reasoning, such as symbol-based reasoning. Most of them were genuinely interested in making sense of chemistry concepts and ideas. However, they seemed to lack important cognitive and metacognitive tools to think more productively about the content. They also frequently failed to take advantage of the collaborative environment that they had created and they did not engage in the co-construction of understandings. In the presence of a more knowledgeable person most students took on a passive role, listening to explanations rather than generating them. These results suggests that instructional and assessment resources need to be enriched and diversified to include questions and problems that require students to think about core concepts and ideas in deeper, more diverse and unfamiliar ways (Raker and Towns, 2010), as well as to self-formulate more thoughtful study questions and problems. On the one hand, in-class time should include well-orchestrated opportunities for sustained engagement in high-level, co-regulated small group discussions that involve students in elaboration of concepts and ideas. These types of events should include explicit reflection on the types of reasoning strategies used by the group to deal with the task as well as on the relationship between group reasoning processes and the quality of learning outcomes.
In general, chemistry instruction at the college level heavily relies on end-of-chapter problems from textbooks as single tools for studying. Unfortunately, these educational resources traditionally target the lower levels of cognitive processing and frequently overemphasize algorithmic versus conceptual problem-solving (Dávila and Talanquer, 2010). Our results suggest that instructors may want to invest more time in the development of alternative educational resources that engage students in diverse modes of reasoning and cognitive processing. In particular, it would be desirable to design multi-component problems that cannot be solved without thoughtful integration of diverse knowledge components and that carefully scaffold student thinking. These types of problems may ask students to, for example, compare and evaluate the strengths and weaknesses of different synthetic approaches to produce a substance or to analyze the composition of a chemical system. These problems may explicitly require students to develop mechanistic models of chemical processes to justify the application of a chemical rule in making a decision. In any case, students need to be confronted with problematic situations that demand the consideration of alternative reasoning strategies to find a plausible solution.
References
- Anderson T. L. and Bodner G. M., (2008), What can we do about “Parker”?: A case study of a good student who didn't “get” organic chemistry, Chem. Educ. Res. Pract., 9, 93–101.
- Anderson L. W. and Krathwohl D. R., (ed.), (2001), A taxonomy for learning, teaching, and assessing: a revision of Bloom's taxonomy of educational objectives, New York: Longman.
- Bhattacharyya G. and Bodner G. M., (2005), “It gets me to the product”: How students propose organic mechanisms, J. Chem. Educ., 82(9), 1402–1407.
- Bransford J. D. and Schwartz D. L., (1999), Rethinking Transfer: A Simple Proposal with Multiple Implications, Rev. Res. Educ., 24, 61–100.
- Bodner G. M. and Herron J. D., (2002), Problem-solving in chemistry. In J. K. Gilbert et al. (ed.), Chemical education: Towards research–based practice (pp. 235–266). Netherlands: Kluwer Academic Publishers.
- Cartrette D. P. and Bodner G. M., (2010), Non-mathematical problem solving in organic chemistry, J. Res. Sci. Teach., 47(6), 643–660.
- Dávila K. and Talanquer V., (2010), Classifying end-of-chapter questions and problems for selected general chemistry textbooks used in the United States. J. Chem. Educ., 87(1), 97–101.
- Eastman C., McCracken M. and Newstetter W. (ed.), (2001), Design knowing and learning: Cognition in design education, Oxford: Elsevier.
- Elrod D. W., Maggiora G. M. and Trenary R. G., (1990), Applications of neural networks in chemistry. 1. Prediction of electrophilic aromatic substitution. J. Chem. Info. Modeling, 30(4), 477–484.
- Entwistle N. J., (2000), Approaches to studying and levels of understanding: the influences of teaching and assessment. In J. C. Smart (Ed.), Higher Education: Handbook of Theory and Research (Vol. XV) (pp. 156–218). New York: Agathon Press.
- Erduran S. and Duschl R., (2004), Interdisciplinary characterizations of models and the nature of chemical knowledge in the classroom, Stud. Sci. Educ., 40, 105–138.
- Ferguson R. and Bodner G. M., (2008), Making sense of the arrow-pushing formalism among chemistry majors enrolled in organic chemistry, Chem. Educ. Res. Pract., 9, 102–113.
- Flippo R. F. and Caverly D. C., (2008), Handbook of college reading and study strategy research, 2nd Edition. New York: Routledge.
- Gabel D. and Bunce D. M., (1994), Research on chemistry problem solving. In D. Gabel (Ed.), Handbook of Research on Teaching and Learning Science. New York: MacMillan.
- Gelernter H., Rose J. R. and Chen C., (1990), Building and refining a knowledge base for synthetic organic chemistry via the methodology of inductive machine learning, J. Chem. Info. Modeling, 30(4), 492–504.
- Gilbert J. K., De Jong O., Justi R., Treagust D. and van Driel J. (ed.), (2002), Chemical education: Towards research-based practice, Dordrecht: Kluwer.
- Gilovich T., Griffin D. and Kahneman D. (ed.), (2002), Heuristics and biases: The psychology of intuitive judgment, Cambridge, England: Cambridge University Press.
- Heyworth R. M., (1999), Procedural and conceptual knowledge of expert and novice students for the solving of a basic problem in chemistry, Int. J. Sci. Educ., 21(2), 195–211.
- Jensen W. B., (1998), Logic, history, and the chemistry textbook. I. Does chemistry have a logical structure? J. Chem. Educ., 75(6), 679–687.
- Justi R. and Gilbert J., (2002), Models and modelling in chemical education. In J. K. Gilbert, O. De Jong, R. Justi, D. F. Treagust, J. H. and van Driel. (ed.), Chemical education: Towards research-based practice (pp 47–68). Dordrecht: Kluwer.
- Kind V., (2004), Beyond Appearances: Students' Misconceptions about Basic Chemical Ideas, London: Royal Society of Chemistry.
- Kolodner J., (1993), Case-based reasoning, San Mateo, CA: Morgan Kaufmann.
- Kovac J., (2002), Theoretical and practical reasoning in chemistry, Found. Chem., 4, 163–171.
- Kozma R. and Russell J., (1997), Multimedia and understanding: Expert and novice responses to different representations of chemical phenomena, J. Res. Sci. Teach., 34, 949–968.
- Kraft A., Strickland A. M. and Bhattacharyya G., (2010), Reasonable reasoning: Multi-variate problem-solving in organic chemistry, Chem. Educ. Res. Pract., 11, 281–292.
- Krathwohl D. R., (2002), A revision of Bloom's taxonomy: A review. Theor. Pract., 42(4), 212–218.
- Leake D. B., (1996), CBR in context: The present and future. In D. Leake (Ed.), Case-based reasoning: Experiences, lessons, and future directions (pp. 1–35). Menlo Park: AAAI Press/MIT Press.
- Maeyer J. and Talanquer V., (2010), The role of intuitive heuristics in students' thinking: Ranking chemical substances, Sci. Educ., 94, 963–984.
- Marton F. and Säljö R., (1997), Approaches to learning. In Marton, F. Hounsell, D. and Entwistle, N.J. (ed.) The Experience of Learning (pp. 39–58). Edinburgh: Scottish Academic Press.
- McClary L. and Talanquer V., (2011), Heuristic reasoning in chemistry: Making decisions about acid strength. Int. J. Sci. Educ., 10 (33), 1433–1454.
- Nurrenbern S. and Pickering M., (1987), Concept learning vs. problem solving: Is there a difference? J. Chem. Educ., 64(6), 508–510.
- Raker, J. R., and Towns, M. H., (2010), Benchmarking Problems Used in Second Year Level Organic Chemistry Instruction, Chem. Educ. Res. Pract., 11, 25–32.
- Schwartz D. L. and Black J. B., (1996), Shuttling between depictive models and abstract rules, Cognitive Sci., 20(4), 457–497.
- Schummer J., (1998), The chemistry core of chemistry: A conceptual approach, HYLE, 4(2), 129–162.
- Smith K. C., Nakhleh M. and Lowery-Bretz S., (2010), An expanded framework for analyzing general chemistry exams, Chem. Educ. Res. Pract., 11, 147–153.
- Stains M. and Talanquer V., (2007), Classification of Chemical Substances Using Particulate Representations of Matter: An Analysis of Student Thinking, Int. J. Sci. Educ., 29(5), 643–661; Erratum 29(7), 935 (2007).
- Stains M. and Talanquer V., (2008), Classification of Chemical Reaction: Stages of Expertise, J. Res. Sci. Teach., 45(7), 771–793.
- Strickland A. M., Kraft A. and Bhattacharyya G., (2010), What happens when representations fail to represent? Graduate students' mental models of organic chemistry diagrams, Chem. Educ. Res. Pract., 11, 293–301.
- Taber K., (2002), Chemical misconceptions –prevention, diagnosis and cure. Vol I: Theoretical background, London: Royal Society of Chemistry.
- Taber K. S. and Bricheno P. A., (2009), Coordinating procedural and conceptual knowledge to make sense of word equations: Understanding the complexity of a 'simple' completion task at the learner's resolution, Int. J. Sci. Educ., 31, 2021–2055.
- Taber K. S. and García-Franco. A., (2010), Learning processes in chemistry: Drawing upon cognitive resources to learn about the particulate structure of matter, J. Learn. Sci., 19, 99–142.
- Talanquer V., (2006), Common sense chemistry: A model for understanding students' alternative conceptions. J. Chem. Educ., 83(5), 811–816.
- Talanquer V., (2011), Macro, Submicro, and Symbolic: The many faces of the chemistry ‘‘triplet’’, Int. J. Sci. Educ., 33(2), 179–195.
- Tang K. C. C., (1993), Spontaneous collaborative learning: A new dimension in student learning experience? High. Educ. Res. Dev., 12(2), 115–130.
- Treagust D. F., Chittleborough G. D. and Mamiala T. L., (2004), Students' understanding of the descriptive and predictive nature of teaching models in organic chemistry, Res. Sci. Educ., 34(1), 1–20.
- Vosniadou S., (1994), Capturing and modeling the process of conceptual change, Learn. Instr., 4, 45–69.
- Winne P. H. and Jamieson-Noel D. L., (2002), Exploring students' calibration of self-reports about study tactics and achievement, Contemp. Educ. Psychol., 27, 551–572.
- Zoller U. and Tsaparlis G., (1997), Higher and lower-order cognitive skills: the case of chemistry, Res. Sci. Educ., 27, 117–130.
| This journal is © The Royal Society of Chemistry 2012 |
