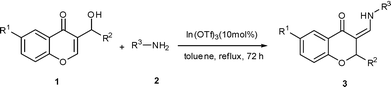
In(III)-Catalyzed tandem reaction of chromone-derived Morita–Baylis–Hillman alcohols with amines
Chen
Wu
ab,
Yuliang
Liu
ab,
Hao
Zeng
a,
Li
Liu
*a,
Dong
Wang
a and
Yongjun
Chen
a
aBeijing National Laboratory for Molecular Sciences (BNLMS), CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: lliu@iccas.ac.cn; Fax: +86 6255 4449
bGraduate School of Chinese Academy of Sciences, Beijing 100049, China
First published on 3rd November 2010
Abstract
The reaction of chromone-derived cyclic Morita–Baylis–Hillman alcohols with amines catalyzed by In(OTf)3 in a one pot process was developed for the convenient and efficient synthesis of 2-substituted-3-aminomethylenechromans. The tandem allylic amination/chromen ring-opening/Michael cyclization reactions were involved in this protocol.
Introduction
Morita–Baylis–Hillman (MBH) adducts, which possess both allylic hydroxyl and Michael acceptor units in the identical molecule, have been illustrated as valuable synthons and starting materials for the synthesis of various biologically active molecules.1–2 Recently MBH adduct as an electrophilic substrate has achieved fruitful results in the allylic substitution reactions with various nucleophiles, including C-nucleophiles, such as arenes and hetero-nucleophiles, such as the compounds bearing –OH, –SH and –NH groups.3 Among them, the carbon–nitrogen bond formation by N-nucleophilic substitution plays an important role for the diversity of synthetic compounds with biological activities.1–3 Generally, the MBH acetate was employed in the nucleophilic substitutions with amines, because hydroxyl group was usually considered as an inefficient leaving group. However, the advantages of directly using allyl alcohol as an allylic reagent were noteworthy: no further functionalization was required for the activation of hydroxyl group and water was the sole by-product after the reaction.4 Although many synthetic methods could be employed in performing the allylic amination of MBH acetates, the N-nucleophilic substitution of MBH alcohols was seldom reported.5 The reaction of MBH adducts with amines could proceed through two ways: either conjugate addition (Scheme 1, path B),5a–e or allylic amination accompanied with dehydration affording α- and γ-products (Scheme 1, path A).5f–h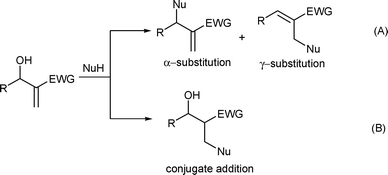 | ||
| Scheme 1 The reaction of MBH alcohols with N-nucleophile. | ||
Benzopyranyl structural moiety is a common heterocyclic framework that can be found in numerous natural compounds and pharmaceutical molecules.6 3-Aminomethylchromons and their derivatives 3-aminomethylenechromans (Fig. 1) have shown important biological activities,7 such as nonsteroidal aromatase inhibitors, antimicrobial effects, and are also interesting as scaffolds in the synthesis of versatile benzopyranyl heterocycle libraries in drug discovery.8 As the Morita–Baylis–Hillman reaction is becoming an environmentally benign and important C–C bond formation reaction, the application of the allylic amination of chromone-derivatised MBH adducts could be efficient methods to construct diverse 3-aminomethylchromone derivatives.
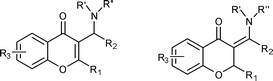 | ||
| Fig. 1 3-Aminomethylchromon derivatives. | ||
Although chromone-derived MBH adducts, which exhibited a cyclic structural unit of MBH adduct, have been used in the synthesis of many heterocycles such as quinoline derivatives and azopinoindoles,9 the allylic amination of such substrates has been seldom reported. As an expansion of our research on the nucleophilic substitution of cyclic MBH adduct, we describe herein the preliminary results of the reaction between chromone-derived MBH alcohols and amines in a one pot process, in which an unexpected tandem allylamination/ring-opening/Michael cyclization reactions was observed to provide 3-aminomethylenechromans in high yields.
Results and discussion
An initial investigation was performed by employing 3-(hydroxy(phenyl)methyl)-chromone 1a and aniline 2a as substrates in the presence of the catalyst In(OTf)3 (10 mol%) in refluxing THF. To our surprise, unlike the reactions of the Morita–Baylis–Hillman alcohols derived from cycloenone with amines,5f this protocol gave neither α- nor γ-products eventually, but 2-phenyl-3-aminomethylene-chroman 3a in 28% yield (Scheme 2). The experimental results for screening solvent and catalyst are summarized in Table 1. A quantitative yield of 3a can be achieved when toluene was used instead of THF (Table 1, entry 2). Several other Lewis acids were screened. In all cases the product 3a was obtained, but in decreased yields. (Table 1, entries 5–8). No reaction occurred either at room temperature or without catalyst, and the starting materials were recovered (Table 1, entries 9 and 10). Water was also used as a solvent but the yield was decreased to 61% (Table 1, entry 11).| Entry | Catalyst | Solvent | T/°C | Yield of 3a (%) |
|---|---|---|---|---|
| a 1a (0.2 mmol), 2a (0.3 mmol), catalyst (0.02 mmol), solvent (2 mL), 72 h. b Without catalyst. | ||||
| 1 | In(OTf)3 | THF | Reflux | 28 |
| 2 | In(OTf)3 | Toluene | Reflux | >99 |
| 3 | In(OTf)3 | MeCN | Reflux | 41 |
| 4 | In(OTf)3 | DCM | Reflux | Trace |
| 5 | Sc(OTf)3 | Toluene | Reflux | 65 |
| 6 | FeCl3 | Toluene | Reflux | 69 |
| 7 | AgOTf | Toluene | Reflux | 90 |
| 8 | InBr3 | Toluene | Reflux | 16 |
| 9 | —b | Toluene | Reflux | 0 |
| 10 | In(OTf)3 | Toluene | r.t. | 0 |
| 11 | In(OTf)3 | Water | Reflux | 61 |
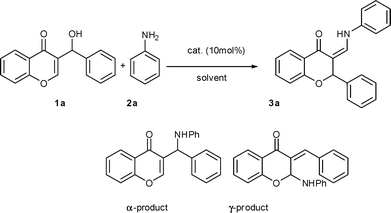 | ||
| Scheme 2 Reaction of 1a with 2a catalyzed by Lewis acid. | ||
As shown in Table 2, the substrate scope of this tandem reaction is quite broad. An array of anilines (2b–2f) with both electron-withdrawing and electron-donating substituents were employed in the reactions with MBH alcohol 1a under the catalysis of In(OTf)3. Good to excellent yields (78–99%) were obtained (entries 2–6). Besides, several aliphatic amines with either acyclic (2g–2i) or cyclic (2j) substituent were suitable to this reaction, affording the corresponding products (3g–3j) in moderate yields (entries 7–10).
|
|
|||
|---|---|---|---|
| Entry | MBH Alcohol (R1,R2) | Amine (R3) | Yield of 3 (%) |
| a MBH alcohol 1 (0.2 mmol), amine 2 (0.3 mmol), In(OTf)3 (0.02 mmol). | |||
| 1 |
1a, R1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) H, R2 H, R2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Ph Ph |
2a, Ph | 3a, 99 |
| 2 | 1a | 2b, 4-ClC6H4 | 3b, 99 |
| 3 | 1a | 2c, 4-BrC6H4 | 3c, 99 |
| 4 | 1a | 2d, 4-F-C6H4 | 3d, 78 |
| 5 | 1a | 2e, 4-MeOC6H4 | 3e, 92 |
| 6 | 1a | 2f, 4-Me-C6H4 | 3f, 82 |
| 7 | 1a | 2g, Bn | 3g, 68 |
| 8 | 1a | 2h, n-C8H17 | 3h, 75 |
| 9 | 1a | 2i, n-C12H25 | 3i, 66 |
| 10 | 1a | 2j, Cy | 3j, 60 |
| 11 |
1b, R1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) H,R2 = 2-BrC6H4 H,R2 = 2-BrC6H4 |
2a | 3k, 82 |
| 12 |
1c, R1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) H,R2 = 4-ClC6H4 H,R2 = 4-ClC6H4 |
2a | 3l, 83 |
| 13 |
1d, R1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Me, R2 Me, R2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Ph Ph |
2a | 3m, 99 |
| 14 |
1e, R1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) H,R2 = n-C3H7 H,R2 = n-C3H7 |
2a | 3n, 55 |
| 15 |
1f, R1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) H,R2 H,R2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Cy Cy |
2a | 3o, 85 |
| 16 |
1g, R1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) H,R2 H,R2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C6H5(CH2)2 C6H5(CH2)2 |
2a | 3p, 65 |
| 17 | 1e | 2g | 3q, 68 |
| 18 | 1g | 2g | 3r, 58 |
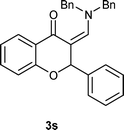
| 19 | 1a | 2k, Bn2NH | 3s, 78 |
Subsequently, we examined the chromone-derived MBH alcohols bearing different functional groups. Importantly, not only MBH alcohols (1b–1d) derived from aromatic aldehydes can undergo the tandem reaction smoothly to provide corresponding products, 2-aryl-3-aminomethylenechromanes (3k–3m), in very good yields (Table 2, entries 11–13), the ones 1e–1g derived from aliphatic aldehydes were also suitable to the tandem reaction, albeit in slightly decreased yields (Table 2, entries 14–16). Furthermore, the MBH alcohols 1e and 1g can also react with benzylamine (2g) to give desired products (3q–3r) in moderate yields (Table 2, entries 17–18). When secondary amine, dibenzylamine (2k) was used in the reaction with 1a under the same conditions, the tandem reaction product 3s was also obtained in 78% yield (entry 19).
The molecular structure of the tandem reaction product 3 was further confirmed by X-ray crystallographic analysis of 3k (Fig. 2).10 The distance between NH and carbonyl is 2.014 Å, which falls into the range of hydrogen bonding interaction.
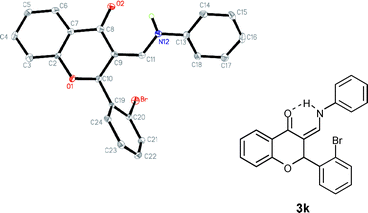 | ||
| Fig. 2 ORTEP drawing of 3k with thermal ellipsoids at 30% probability levels. | ||
Based on the results of X-ray analysis, cis-configuration of the newly formed double bond could be determined.
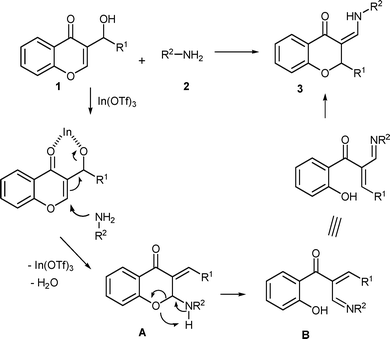
The plausible mechanism of the tandem reaction of MBH alcohol with amine was proposed, and shown in Scheme 3. The MBH alcohol derived from chromone was activated by In(OTf)3. The N-nucleophile attacked γ-position of MBH alcohol to carry out allylic amination reaction accompanied with dehydration, generating an intermediate A. Subsequently, intermediate A. underwent chromone ring-opening9b–c to form an active Michael acceptor B which was followed by intramolecular oxa-Michael cyclization to yield 2-substituted-3-aminomethylenechroman 3.
It was interesting to note that if the reaction of MBH alcohol 1a with diphenylamine 2l was carried out under the catalysis of In(OTf)3, the α-regioselective product of Friedel–Crafts alkylation (4) was formed in 43% yield, as well as unexpected indenone 5 was obtained in 38% yield via cascade allylic amination/chromen ring-opening/C-nucleophilic Friedel–Crafts cyclization (Scheme 4).
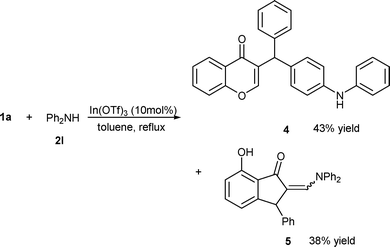 | ||
| Scheme 4 Reaction of 1a with diphenylamine 2l. | ||
Conclusions
In summary, we developed the reaction of chromone-derived Morita–Baylis–Hillman alcohols with amines under In(OTf)3 catalysis for the synthesis of 2-substituted-3-aminomethylene-chromans. This protocol combined allylic amination, ring-opening, Michael cyclization reactions in a one pot process. A tentative mechanism for the tandem reactions was proposed. Investigation of the tandem reactions and their application in the synthesis of biologically active compounds is ongoing.Experimental
General Methods
The 1H and 13C NMR spectra were recorded on Bruker-AV 300 spectrometer and chemical shift reported in CDCl3 or DMSO-d6 with tetramethylsilane as an internal standard. IR spectra were recorded on a Bruker tensor 27 infrared spectrometer. HRMS spectra were recorded on GCT-Mass Micromass spectrometer. Melting points were measured on Beijing-Tiker X-4 apparatus without correction. All reactions were carried out in oven dried flasks. Common reagents were purchased from commercial sources and were used without further purification. The Morita–Baylis–Hillman alcohols were prepared according to literature methods.11 All reactions were performed under nitrogen atmosphere.Typical experimental procedures for the reaction of MBH alcohol with amine
To a stirred solution of 3-(hydroxy(phenyl)methyl)-chromone (1a, 51 mg, 0.2 mmol) in toluene (2 mL) at room temperature was added aniline (2a, 35 μL, 0.3 mmol) and In(OTf)3 (11 mg, 0.02 mmol) sequentially and the reaction mixture was heated to reflux for 72 h. Upon completion of the reaction as judged by TLC, the reaction was quenched with water and extracted with DCM twice. The combined extract was dried over anhydrous Na2SO4 and concentrated in vacuo. The crude product was purified by flash chromatography on silica gel (eluent: EtOAc/petroleum ether = 1/8) to afford the product 3a.Acknowledgements
We thank the National Natural Science Foundation of China, Ministry of Science and Technology (No. 2009ZX09501-006) and the Chinese Academy of Sciences for the financial support.References
- For reviews of MBH reactions, see: (a) D. Basavaiah, A. J. Rao and T. Satyanarayana, Chem. Rev., 2003, 103, 811 CrossRef CAS; (b) Y. L. Shi and M. Shi, Org. Biomol. Chem., 2007, 5, 1499 RSC; (c) D. Basavaiah, K. V. Rao and R. J. Reddy, Chem. Soc. Rev., 2007, 36, 1581 RSC; (d) G. Masson, C. Housseman and J. P. Zhu, Angew. Chem., Int. Ed., 2007, 46, 4614 CrossRef CAS.
- For reviews of aza-MBH reactions, see: (a) V. Declerch, J. Martinez and F. Lamaty, Chem. Rev., 2009, 109, 1 CrossRef CAS; (b) Y. Shi and M. Shi, Eur. J. Org. Chem., 2007, 2905 CrossRef CAS.
- For selected C-nucleophiles, see: (a) X. Zhang, W. Rao, S. W. H. Chan and P. W. H. Chan, Org. Biomol. Chem., 2009, 7, 4186 RSC; (b) H. L. Cui, J. Peng, X. Feng, W. Du, K. Jiang and Y. C. Chen, Chem.–Eur. J., 2009, 15, 1574 CrossRef CAS; (c) G. W. Kabalka, G. Dong, B. Venkataiah and C. Chen, J. Org. Chem., 2005, 70, 9207 CrossRef CAS; (d) S. M. Ma, S. C. Yu, Z. H. Peng and H. Guo, J. Org. Chem., 2006, 71, 9865 CrossRef CAS; (e) M. L. Kantam, K. B. S. Kumar and B. Sreedhar, J. Org. Chem., 2008, 73, 320 CrossRef CAS; (f) Y. Q. Jiang, Y. L. Shi and M. Shi, J. Am. Chem. Soc., 2008, 130, 7202 CrossRef CAS; (g) P. V. Ramachandran, S. Madhi, L. Bland-Berry, M. V. R. Reddy and M. J. O'Donnell, J. Am. Chem. Soc., 2005, 127, 13450 CrossRef CAS; (h) S. K. Mandal, M. Paira and S. C. Roy, J. Org. Chem., 2008, 73, 3823 CrossRef CAS; (i) S. H. Kim, K. H. Kim, H. S. Kim and J. N. Kim, Tetrahedron Lett., 2008, 49, 1948 CrossRef CAS; (j) V. Singh, G. P. Yadav, P. R. Maulik and S. Batra, Tetrahedron, 2008, 64, 2979 CrossRef CAS; (k) For O-nucleophiles, see: B. M. Trost, O. R. Thiel and H. C. Tsui, J. Am. Chem. Soc., 2003, 125, 13155 Search PubMed; (l) C. R. Reddy, N. Kiranmai, G. S. K. Babu, G. D. Sarma, B. Jagadeesh and S. Chandrasekhar, Tetrahedron Lett., 2007, 48, 215 CrossRef CAS; (m) H. S. Kim, S. Gowrisankar, S. H. Kim and J. N. Kim, Tetrahedron Lett., 2008, 49, 3858 CrossRef CAS; (n) E. Ramesh and R. Raghunathan, Tetrahedron Lett., 2008, 49, 1125 CrossRef CAS; (o) For S-nucleophiles, see: A. Kamimura, R. Morita, K. Matsuura, Y. Omata and M. Shirai, Tetrahedron Lett., 2002, 43, 6189 Search PubMed; (p) For N-nucleophiles, see: C. G. Lee, K. Y. Lee, S. Lee and J. N. Kim, Tetrahedron, 2005, 61, 1493 Search PubMed; (q) H. L. Cui, X. Feng, J. Peng, J. Lei, K. Jiang and Y. C. Chen, Angew. Chem., Int. Ed., 2009, 48, 5737 CrossRef CAS; (r) R. Pathak, S. Madapa and S. Batra, Tetrahedron, 2007, 63, 451 CrossRef CAS; (s) C. W. Cho, J. R. Kong and M. J. Krische, Org. Lett., 2004, 6, 13379; (t) V. Singh and S. Batra, Tetrahedron, 2008, 64, 4511 CrossRef CAS and references cited therein.
- For direct substitution of π-alcohols, see: (a) M. Bandini and M. Tragni, Org. Biomol. Chem., 2009, 7, 1501 RSC; (b) N. Ljungdahl and N. Kann, Angew. Chem., Int. Ed., 2009, 48, 642 CrossRef CAS; (c) C.-J. Li; and B. M. Trost, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 13197 CrossRef CAS; (d) J. A. McCubbin, H. Hosseini and O. V. Krokhin, J. Org. Chem., 2010, 75, 959 CrossRef CAS.
- (a) S. Nag, G. P. Yadav, P. R. Maulik and S. Batra, Synthesis, 2007, 911 CAS; (b) M. K. Kundu and S. V. Bhat, Synth. Commun., 1999, 29, 93 CrossRef CAS; (c) R. Pathak, K. R. Amrendra and S. Batra, Synlett, 2005, 5, 848; (d) R. Phthak and S. Batra, Tetrahedron, 2007, 6, 9448; (e) S. Nag, R. Pathak, M. Kumar, P. K. Shukla and S. Batra, Bioorg. Med. Chem. Lett., 2006, 16, 3824 CrossRef CAS; (f) Y. L. Liu, L. Liu, D. Wang and Y. J. Chen, Tetrahedron, 2009, 65, 3473 CrossRef CAS; (g) K. Y. Lee, H. S. Lee and J. N. Kim, Bull. Korean Chem. Soc., 2008, 29, 1099 CAS; (h) S. Rajesh, B. Banerji and J. Iqbal, J. Org. Chem., 2002, 67, 7852 CrossRef CAS.
- (a) G. P. Ellis, Chromenes, chromanones, and chromones, Wiley-Interscience: New York, 1977 Search PubMed; (b) H. Miao and Z. Yang, Org. Lett., 2000, 2, 1765 CrossRef CAS; (c) R. S. Varma, J. Heterocycl. Chem., 1999, 36, 1565 CrossRef CAS; (d) K. C. Nicolaou, J. A. Pfefferkorn, A. J. Roecker, G.-Q. Cao, S. Barluenga and H. J. Mitchell, J. Am. Chem. Soc., 2000, 122, 9939 CrossRef CAS; (e) J. R. S. Hoult, M. A. Moroney and M. Paya, Methods Enzymol., 1994, 234, 443 CAS.
- (a) F. E. Ward, D. L. Garling and R. T. Buckler, J. Med. Chem., 1981, 24, 1073 CrossRef CAS; (b) M. Recanatini, A. Bisi, A. Cavalli, F. Belluti, S. Gobbi, A. Rampa, P. Valent, M. Palzer, A. Palusczak and R. W. Hartmann, J. Med. Chem., 2001, 44, 672 CrossRef CAS; (c) A. Cavalli, A. Bisi, C. Bertucci, C. Rosini, A. Paluszcak, S. Gobbi, E. Giorgio, A. Rampa, F. Belluti, L. Piazzi, P. Valenti, R. W. Hartmann and M. Recanatini, J. Med. Chem., 2005, 48, 7282 CrossRef CAS; (d) S. Gobbi, A. Cavalli, A. Rampa, F. Belluti, L. Piazzi, A. Paluszcak, R. W. Hartmann, M. Recanatini and A. Bisi, J. Med. Chem., 2006, 49, 4777 CrossRef CAS; (e) E. A. A. Wallen, K. Dahlen, M. Grotli and K. Luthman, Org. Lett., 2007, 9, 389 CrossRef CAS; (f) Y. P. Luo and G. F. Yang, Bioorg. Med. Chem., 2007, 15, 1716 CrossRef CAS.
- (a) O. Bruno, S. Schenone, A. Ranise, F. Bondavalli, E. Barocelli, V. Ballabeni, M. Chiavarini, S. Bertoni, M. Tognolini and M. Impicciatore, Bioorg. Med. Chem., 2001, 9, 629 CrossRef CAS; (b) O. Bruno, C. Brullo, A. Ranise, S. Schenone, F. Bondavalli, E. Barocelli, V. Ballabeni, M. Chiavarini, M. Tognolini and M. Impicciatore, Bioorg. Med. Chem. Lett., 2001, 11, 1397 CrossRef CAS; (c) H. An, S. J. Eum, M. Koh, S. K. Lee and S. B. Park, J. Org. Chem., 2008, 73, 1752 CrossRef CAS.
- (a) D. Basavaiah and A. J. Rao, Tetrahedron Lett., 2003, 44, 4365 CrossRef CAS; (b) D. Basavaiah, R. J. Reddy and J. S. Rao, Tetrahedron Lett., 2006, 47, 73 CrossRef CAS; (c) Z. Shafiq, L. Liu, Z. Liu, D. Wang and Y. J. Chen, Org. Lett., 2007, 9, 2525 CrossRef CAS.
- CCDC 784290 contains the supplementary crystallographic data for 3k. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre viawww.ccdc.cam.ac.uk/datarequest/cif, and are in the ESI. Crystal, data for compound 3k: C22H16BrNO2, M = 406.27, Monoclinic, a = 15.7458(9) Å, α = 90°, b = 5.1545(2) Å, β = 92.048(2)°, c = 20.8004(10) Å, γ = 90°, V = 1687.12(14) Å 3, T = 173(2) K, space group = P21/c, Z = 4, Number of Reflections = 6168, Independent reflections = 3846, [R(int) = 0.0554], Final R indices [I > 2δ(I)] R1 = 0.0850, wR2 = 0.1138, R indices (all data) R1 = 0.1359, wR2 = 0.1247.
- (a) S. Luo, P. G. Wang and J. P. Cheng, J. Org. Chem., 2004, 69, 555 CrossRef CAS; (b) S. Luo, X. Mi, P. G. Wang and J. P. Cheng, J. Org. Chem., 2004, 69, 8413 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |