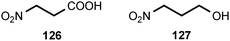Naturally-occurring nitro compounds
Ronald
Parry
*a,
Shirley
Nishino
b and
Jim
Spain
b
aDepartment of Chemistry, Rice University, Houston, Texas, USA. E-mail: Parry@rice.edu
bDepartment of Civil and Environmental Engineering, Georgia Institute of Technology, Atlanta, Georgia, USA
First published on 3rd December 2010
Abstract
Covering: up to the end of 2010
Naturally-occurring nitro compounds display great structural diversity, and a wide range of biological activities. This review summarizes current information on the structures of naturally-occurring nitro compounds and on the biosynthesis of the nitro group.
 Ronald Parry | Ronald J. Parry was born in Los Angeles, CA. He received his Ph.D. from Brandeis University, working under the guidance of Professor James B. Hendrickson. He then spent two years studying alkaloid biosynthesis with Professor Sir Alan Battersby. This was followed by one year of postdoctoral work in the laboratory of Professor William S. Johnson. After six years as an Assistant Professor at Brandeis University, he moved to Rice University in 1978 where he became full professor in 1982. His research has focused on the biosynthesis of natural products, with an emphasis on compounds containing N–N bonds and naturally occurring sulfur compounds. |
 Shirley Nishino | Shirley Nishino received an M.S. in zoology from the University of California, Davis. She joined Jim Spain at the Air Force Research Laboratory in 1985. She is currently a research scientist working with Jim Spain at the Georgia Institute of Technology. She has specialized in discovery of organisms that grow on synthetic and naturally nitrated compounds, and deciphering the catalytic pathways. |
 Jim Spain | Jim Spain received a Ph.D. in microbiology from The University of Texas at Austin. He worked for five years at the U.S. Environmental Protection Agency Marine Environmental Research Laboratory, before joining the Air Force Research Laboratory in 1985. He is currently a professor in Civil and Environmental Engineering at the Georgia Institute of Technology. For the past 30 years he has studied the mechanisms of biodegradation of synthetic organic compounds. He is a former editor for Applied and Environmental Microbiology and has published extensively on the biodegradation of synthetic organic compounds. |
1 Introduction
Natural products containing nitro groups have been isolated from plants, fungi, bacteria, and mammals. These compounds display great structural diversity, and they exhibit a wide range of biological activities including antibiotic, antitumor, and cell signaling properties.Some attention has been directed toward understanding the biosynthesis of nitro groups. Three mechanisms have been reviewed by Winkler and Hertweck.1 Some nitro compounds arise through the oxidation of an amino group, as has been established for the aromatic nitro groups of pyrrolnitrin and aureothin (section 3) and the aliphatic nitro group of the sugar D-rubranitrose (section 5). A second mechanism for nitration involves the action of a nitric oxide synthase during the biosynthesis of the thaxtomins (section 4). A third mechanism appears to involve the direct introduction of a nitro group via an electrophilic nitration reaction. This mechanism may account for the formation of the antibiotic dioxopyrrolomycin (section 2). The structural variety exhibited by the family of naturally occurring nitro compounds suggests that additional mechanisms for nitro group formation may remain to be discovered.
Many of the over 200 naturally occurring nitro compounds were identified while screening for antibiotic and other medicinal properties. In most cases, the physiological and ecological roles of these compounds have not been investigated. A recent review2 provides an overview of natural nitroaromatic compounds and the mechanisms involved in their biodegradation.
This review surveys the structures and biological activities of the naturally occurring nitro compounds isolated to date, and it summarizes the available information on the biosynthesis of nitro groups.
2 Monocyclic nitrated aromatic compounds
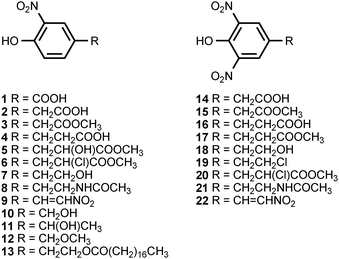
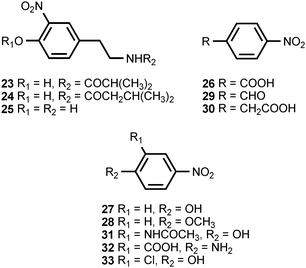
The most prolific natural source of monocyclic, nitrated aromatic compounds is the psychrotolerant, Gram-negative bacterium Salegentibacter sp. strain T436, which is found in Arctic pack ice. Thus far, a total of 21 monocyclic, nitroaromatic compounds (1–9, 14–22, 27, 34, 35) have been isolated from cultures of this microorganism grown on complex media dissolved in artificial sea water.3,4 The fermentations were optimized for production of bioactive, secondary metabolites,4 and it is not known whether the bacterium produces the compounds in its native habitat. A number of these compounds exhibit antimicrobial and cytotoxic activities, with 2-nitro-4-(2′-nitroethenyl)phenol (9) being the most potent. Monocyclic nitroaromatic compounds have also been isolated from a wide range of other living organisms. 4-Hydroxy-3-nitrobenzyl alcohol (10) has been found in the marine bryozoan Phidolophora pacifica and the deuteromycete Pyricularia oryzae.5,6P. pacifica also produces 4-methoxymethyl-2-nitrophenol (12), while P. oryzae produces 4-hydroxy-3-nitrophenethyl alcohol (7), 4-hydroxy-α-methyl-3-nitrobenzyl alcohol (11), 4-hydroxy-3-nitrophenylacetic acid (2), and N-acetyl-4-hydroxy-3-nitrophenethylamine (8).5A facultatively anaerobic, halophilic bacterium isolated from the Great Salt Plains of Oklahoma produces the N-acylated 4-hydroxy-3-nitrophenethylamines 23 and 24 as well as 4-hydroxy-3-nitrophenyl acetic acid (2).7 Unacylated 4-hydroxy-3-nitrophenethylamine (25) has been isolated from the cactus Cereus validus.8 The stearate ester of 4-hydroxy-3-nitrophenethyl alcohol (13) is found in the roots of the vascular plant Bignonia unguis-cati,9 and 4-nitrophenylacetic acid (30) is produced by Pseudomonas fluorescens.10
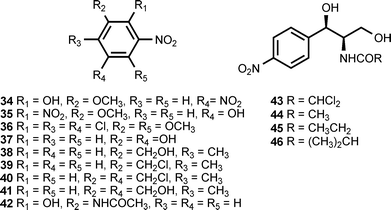
The culture broth of the basidiomycete Lepista diemii contains 4-nitroanisole (28) and 4-nitrobenzaldehyde (29).11 4-Nitrobenzoic acid (26) has been detected in cultures of Streptomyces thioluteus, where it is presumed to be an intermediate in the biosynthesis of aureothin (71) (see section 3).12 The biotransformation of the phytoanticipins 2-benzoxazolininone (BOA) and 2-hydroxy-1,4-benzoxazin-3-one (HBOA) by four endophytic fungi isolated from the vascular plant Aphelandra tetragona produce N-acetyl-2-hydroxy-5-nitroaniline (31) and N-acetyl-2-hydroxy-3-nitroaniline (42).13 5-Nitroanthranilic acid (32) has been isolated from Streptomyces scabies, an organism that also produces thaxtomin A (see section 3).14 The authors could find no link between the biosynthesis of 5-nitroanthranilic acid and the thaxtomins, but suggested that the presence of 32 implied a much earlier nitration step than had previously been hypothesized. The fungus Fomes robiniae produces 1,4-dimethoxy-2-nitro-3,5,6-trichlorobenzene (36).15 5-Nitroresorcinol (37) is produced by the myxobacterial species Stigmatella erecta and Stigmatella aurantiaca.16 The latter organism also produces the monomethyl and dimethyl ethers of 37.16
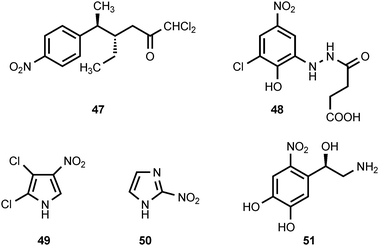
The growth medium of a fungus (B60) isolated from the mangrove Acanthus ilicifolium contains 2-methyl-5-nitrobenzyl alcohol (38), 2-methyl-5-nitrobenzyl chloride (39), 2-methyl-3-chloromethyl-5-nitrobenzyl chloride (40), and 3-hydroxymethyl-2-methyl-5-nitrobenzyl alcohol (41).17 Chloramphenicol (chloromycetin) (43) is an antibiotic produced by Streptomyces venezuelae and several other actinomycetes.18,19 The compound binds to the 50S ribosomal subunit and blocks fundamental ribosomal functions.20 Chloramphenicol is accompanied in the fermentation broth by the congeners corynecin I (44), corynecin II (45), and corynecin III (46). The latter compounds are also produced by Corynebacterium sp. KY 4339.21,22
Chloramphenicol exhibits antibacterial activity against both Gram-positive and Gram-negative bacteria.18 The corynecins show a similar spectrum of antimicrobial activity. 4-Aminophenylalanine appears to be an intermediate in the biosynthetic pathway for the above antibiotics.23 It is currently believed that the aryl nitro group in these antibiotics arises from the oxidation of an aryl amino group.19 This hypothesis is consistent with the origin of the aryl nitro groups in the antibiotics pyrrolnitrin and aureothin (see section 3).
Ayamycin (47) is an antibiotic produced by Nocardia sp. ALAA 2000,24 which was isolated from the marine red alga Laurencia spectabilis collected off the Egyptian, Ras-Gharib coast of the Red Sea. Ayamycin exhibits potent antibacterial activity as well as antifungal activity.24 The carrot truffle, Stephanospora caroticolor, is a rare gasteromycete. Extraction of the young, uninjured fruiting bodies yields stephanosporin (48).25 If the fruiting bodies are stored, dried, or crushed before extraction, then 2-chloro-4-nitrophenol (33) accompanies stephanosporin in the extracts.25 The oxidative transformation of stephanosporin into 2-chloro-4-nitrophenol appears to be an enzymatic process induced by injury of the fruiting body.25 Since 2-chloro-4-nitrophenol displays antifungal activity, the enzymatic formation of 33 from 48 may serve as a defense mechanism for Stephanospora. Pyrrolomycin A (49) is an antibiotic produced by Actinosporangium vitaminophilum SF-2080,26,27 an actinomycete that produces other antibiotics containing a nitrated pyrrole ring (see section 3). Pyrrolomycin A is active against both Gram-positive and Gram-negative bacteria as well as some fungi.27 The antibiotic azomycin (2-nitroimidazole) (50) has been isolated from the culture broths of Nocardia mesenterica,28,29Streptomyces eurocidicus,30 and Pseudomonas fluorescens.31 The compound is active against a variety of bacteria including Mycobacterium tuberculosis.28,30,31 Azomycin is a prodrug whose biological activity results from in vivo reduction of the nitro group to generate more reactive species.32 6-Nitronorepinephrine (51) has been identified in rat brain33 and in rat spinal cord tissue.34 The amount of 6-nitronorepinephrine present in rat brain was reduced by intraperitoneal administration of the nitric oxide synthase inhibitor NG-nitro-L-arginine methyl ester (L-NAME), suggesting that nitric oxide synthase participates in the formation of 6-nitronorepinephrine.33 Nitric oxide synthase has also been implicated in the formation of the thaxtomins (see section 3).
3 Multicyclic nitrated aromatic compounds
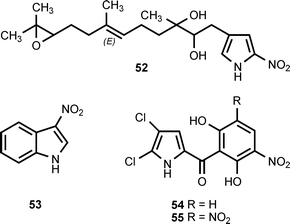
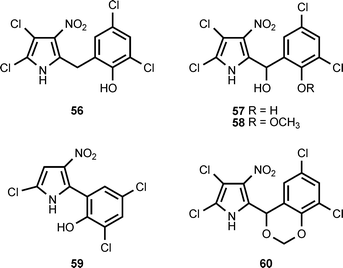
The nitropyrrole 52 is produced by a marine actinomycete, isolated from marine sediments, that falls into the MAR4 clade.35 3-Nitroindole (53) has been isolated from the psychrotolerant Gram-negative bacterium Salegentibacter sp. strain T436.3 3′-Nitropyoluteorin (54) and 3′,5′-dinitropyoluteorin (55) are produced by culturing of Pseudomonas aeruginosa S10B2 in a medium containing n-paraffins.36,37The pyrrolomycin antibiotics are produced by Actinosporangium vitaminophilum, Streptomyces fumanus, and Streptomyces sp. UC11065. The antibiotics in this family include pyrrolomycin A (49) (see section 2), pyrrolomycin B (56), pyrrolomycin E (59), pyrrolomycin G (57), pyrrolomycin H (58), and dioxapyrrolomycin (60).38–42 Although the pyrrolomycin biosynthetic genes clusters have been cloned from A. vitaminophilum and Streptomyces sp. UC11065, analysis of the gene clusters does not reveal the mechanism for introduction of a nitro group into these antibiotics.43 Biosynthetic investigations indicate that the formation of the nitro group does not involve a nitric oxide synthase.44 It has also been reported that (18O3,15N)-nitrate is incorporated into dioxapyrrolomycin without significant loss of the 18O-label.45
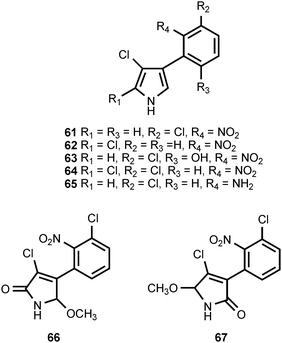
The antibiotic pyrrolnitrin (61) is produced by Pseudomonas species46,47 as well as by Myxococcus fulvus48 and Enterobacter agglomerans.49 Pyrrolnitrin is accompanied by isopyrrolnitrin (62) and oxypyrrolnitrin (63) in Pseudomonas pyrrocinia50,51 and by 2-chloropyrrolnitrin (64) in Burkholderia cepacia (formerly Pseudomonas cepacia).52 Pyrrolnitrin exhibits both antifungal and antibacterial activity.53 The pyrrolonitrin biosynthetic pathway has been shown to require four genes.54 Experiments with deletion mutants have defined the role of the genes in pyrrolonitrin formation and shown that aminopyrrolnitrin (65) is a biosynthetic intermediate.55 These studies also demonstrated that the oxidation of 65 to pyrrolnitrin is catalyzed by the prnD gene product.55 Detailed studies of PrnD have shown that the protein utilizes molecular oxygen as a co-substrate and contains a Rieske [2Fe–2S] iron–sulfur cluster.56 Experiments with substrate analogs suggest that the oxidation of the aryl amino group to a nitro group proceeds through aryl hydroxylamine and aryl nitroso species.57 Two compounds (66, 67), that appear to be formed by oxidation of pyrrolnitrin have been isolated from Burkholderia cepacia K87. Both compounds exhibited only marginal bioactivity.58
Three nitrated isoflavones, 3′-nitrodaidzein (68), 3′-nitrogenistein (69) and 3′,5′-dinitrogeistein (70), have been isolated from cultures of the Arctic ice bacterium Salegentibacter sp. T436.4 These compounds exhibited no significant phytotoxic or antimicrobial activities. They could be derived from the isoflavones daidzein and genistein, which are commonly found in the soybean meal that was present in the Salegentibacter fermentation medium.4
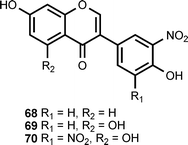
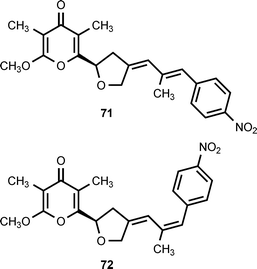
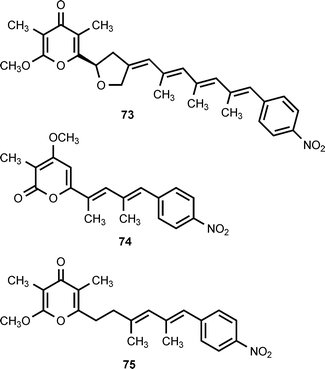
A group of structurally-related nitrophenyl pyrones has been isolated from Streptomyces thioluteus, Streptomyces spectabilis, Streptomyces luteoreticuli, Streptomyces griseus, and Streptomyces sp. MM23. Five compounds have been characterized: aureothin (71),59,60 alloaureothin (72),61 spectinabilin (also known as neoaureothin) (73),62,63 luteoreticulin (also known as griseulin) (74),64–67 and luteothin (also known as deoxyaureothin) (75).60,68 The compounds display a variety of biological activities. Aureothin exhibits antifungal, antitumor, and anti-Helicobacter pylori activity,61 while alloaureothin shows inhibitory activity against human fibrosarcoma HT1080 cells, with an IC50 value of 30 μM.61 Luteoreticulin possesses nematocidal and mosquitocidal activities.67 Investigations of aureothin biosynthesis have shown that the skeleton of this compound is assembled by a type I polyketide synthase (PKS) that contains a module used in an iterative fashion.69,70 The aureothin PKS utilizes 4-nitrobenzoic acid as the polyketide starter unit.12 Results obtained by cloning, sequencing, and analysis of the neoaureothin (spectinabilin) biosynthetic gene cluster have shown that the neoaureothin cluster is remarkably similar to the aureothin cluster. Furthermore, the analysis suggested that the aureothin pathway probably arose from the neoaureothin pathway by gene deletion.71
The aureothin biosynthetic gene cluster contains a gene (aurF) encoding a monooxygenase that utilizes molecular oxygen to catalyze a six-electron oxidation of 4-aminobenzoic acid to 4-nitrobenzoic acid.72,73 4-Hydroxyaminobenzoate appears to be an intermediate in this conversion.73 Investigations of the substrate specificity of this enzyme have shown that it exhibits strict regio- and chemselectivity for an aromatic amino group para to an acidic group.74 Structural and EPR investigations of AurF indicate that active forms of the protein contain either a binuclear manganese cluster,75 a heterodinuclear Mn/Fe cluster,76 or a binuclear iron cluster.77 Spectroscopic studies of the di-iron form of AurF suggest that a peroxo-Fe2III/III intermediate is involved in the oxidation of the amino group of the substrate,78 and that only one equivalent of oxygen is needed to convert the intermediate 4-hydroxyaminobenzoate into 4-nitrobenzoate.79 AurF appears to be unrelated to PrnD, the enzyme that catalyzes the oxidation of aminopyrrolnitrin to pyrrolnitrin.
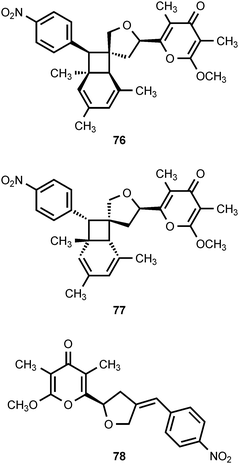
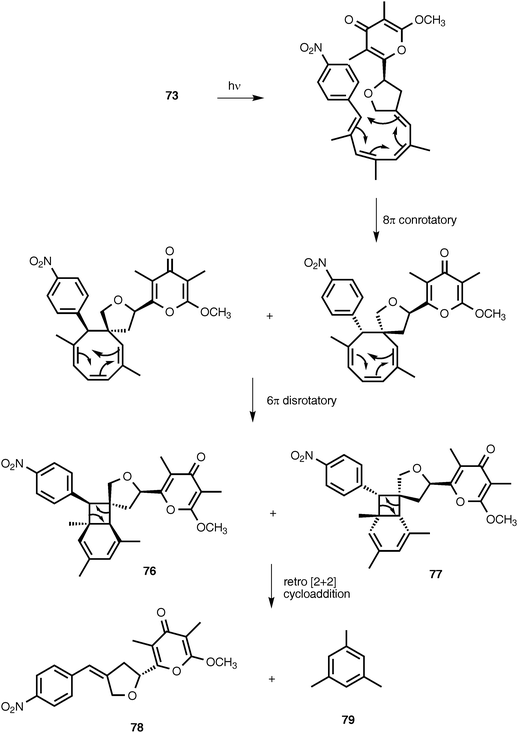
Two novel nitrophenyl pyrones, SNF4435C (76) and SNF4435D (77), have been isolated from the fermentation broth of Streptomyces spectabilis SNF4435. SNF4435C and SNF4435D exhibit no antimicrobial activity against Gram-negative bacteria, but some activity is displayed against Gram-positive bacteria and fungi.80,81 Both SNF4435C and SNF4435D display potent immunosuppressive activity in vitro and selectively suppressed B-cell proliferation induced by lipopolysaccharides.80 Subsequent investigations have revealed that 76 and 77 are derivatives of spectinabilin (73) that are formed as the result of a nonenzymatic, photoinduced E/Z-isomerization–electrocyclization reaction sequence (Scheme 1).82 Furthermore, another nitrophenyl pyrone, orinocin (78), is produced from 76 and 77 by a formal retro-[2 + 2] cycloaddition that also produces mesitylene (79) (Scheme 1).82
 | ||
| Scheme 1 | ||
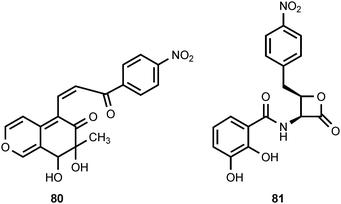
A coumarin derivative (80) containing a 4-nitrophenyl substituent has been isolated along with forty-three other coumarin analogs from marine fungi associated with mangroves. The compound does not exhibit any significant cytotoxicity.83 The novel β-lactone obafluorin (81) is produced by Pseudomonas fluorescens.84,85 Obafluorin displays weak antimicrobial activity. Investigations of obafluorin biosynthesis have established that L-4-aminophenylalanine is an intermediate.10 This finding suggests that the nitro group of obafluorine is derived by oxidation of an aryl amino group.
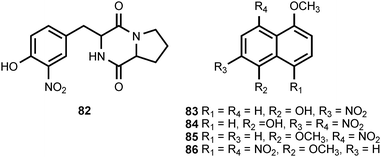
The 2,5-diketopiperazine pyriculamide (82) is produced by the fungus Pyricularia oryzae.5 Its biological activity has not been assessed. The endophytic fungus Coniothryium produces four nitrated naphthalene derivatives (83–86).86 Three of these compounds (83–85) exhibit excellent antifungal activity, while the fourth naphthalene derivative, 86, is inactive. The biosynthetic origin of these compounds is obscure.
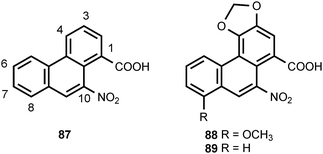
Plants in the family Aristolochiaceae produce a large number of compounds that are derivatives of 10-nitrophenanthrene-1-carboxylic acid (87).87 The structural variations in this family of compounds arise from oxygenation at the C-3, C-4, C-6, C-7, and C-8 positions of the phenanthrene nucleus and by esterification of the C-1 carboxyl group. Three 6-O-glucosyl derivatives of 87 have also been reported. Major constituents of Aristolochia sp. include aristolochic acid I (88) and aristolochic acid II (89). The presence of aristolochic acids in some herbal preparations can cause nephrotoxicity and upper urothelial cancer.88,89 The natural occurrence of aristolochic acid derivatives was surveyed by Kumar et al. in 2003.87 Biosynthetic investigations have shown that aristolochic acid I (88) is derived from tyrosine via the intermediacy of 3,4-dihydroxyphenylalanine, and the 1-benzyltetrahydroisoquinoline alkaloid norlaudanosoline.90–92 Experiments with [β-14C,15N]-tyrosine provided evidence that the nitro group of aristolochic acid I is derived by oxidation of the amino group of tyrosine.90
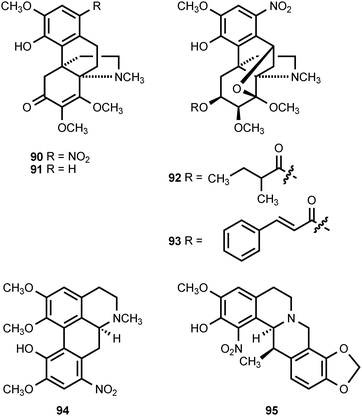
1-Nitroaknadinine (90) and aknadinine (91) co-occur in Stephania sutchuenensis, suggesting that the former compound is derived from the latter.93,94 Both of these compounds are hasubanan alkaloids, which are found exclusively in the genus Stephania (Menispermaceae). The roots of S. sutchuenesis are used in herbal medicine for the treatment of colds, sore throats and arthritic pain.94 The additional nitrated hasubanan alkaloids, stephalonines J (92) and K (93), have been extracted from Stephania longa.95 The whole plant of Stephania longa has been used for the treatment of fever, inflammation, and dysentery.95 The aporphine alkaloid 8-nitroisocorydine (94) has been found in Duguertia furfuracea (Annonaceae),96 while the tetrahydroprotoberberine alkaloid 1-nitroapocavidine (95) has been isolated from the Chinese medicinal plant Corydalis saxicola (Papaveraceae).97
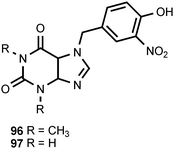
The unusual purine derivatives phidolopin (96) and desmethylphidolopin (97) are found in the marine bryozoan Phidolopora pacifica.6,98 Phidolopin exhibits both antifungal and anti-algal activity6,98 and may serve to prevent fouling by epiphytes.98 Compounds 96 and 97 are found in varying combinations with 10 and 12 in Northeast Pacific bryozoans belonging to five families distributed among the two classes of marine bryozoans. The authors found the widespread biosynthesis of the same nitro compounds by not very closely related species improbable, and speculated that the nitro compounds extracted from the bryozoans were of microbial origin.6
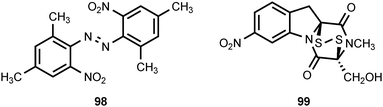
The leaves of Aconitum sungpanense have been reported to contain trans-2,2′,4,4′-tetramethyl-6,6′-dinitroazobenzene (98).99 The biological activity of this unusual compound has not been examined. A co-culture of a mine drainage-derived bacterial strain Sphingomonas KMK-001 with a mine drainage-derived fungal strain Aspergillus fumigatus KMC-901 resulted in the production of the 2,5-diketopiperazine disulfide glionitrin A (99).100 The compound could not be detected in monocultures of either KMK-001 or KMC-901. Glionitrin A exhibited significant antimicrobial activity as well as cytotoxic activity against four human cancer cell lines.100
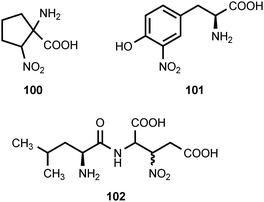
4 Nitrated amino acids and peptides
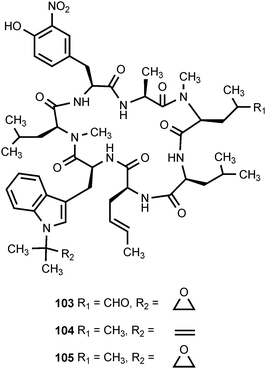
The optically-inactive amino acid 1-amino-2-nitrocyclopentanecarboxylic acid (100) is produced by Aspergillus wentii.101 In higher plants, this compound produces loss of apical dominance and inhibits mitosis in root tips and apical buds. The inhibition caused by 100 is reversed by L-leucine.102 3-Nitrotyrosine (101) is found in a wide spectrum of proteins as the result of a posttranslational modification that occurs in a number of acute or chronic pathological conditions.103,104 The nitration of protein tyrosine residues is a selective process where only some proteins become nitrated and only one or a few tyrosine residues are modified in each protein. The in vivo nitration of tyrosine residues is believed to involve two steps.104 The first step is oxidation of the phenolic hydroxyl group of tyrosine to produce a phenoxy radical. This is followed by a reaction between the phenoxy radical and nitrogen dioxide (˙NO2). The nitrogen dioxide can be produced by homolytic cleavage of peroxynitrite (HOONO) derived from the reaction of nitric oxide (˙NO) with superoxide (O2−˙) or from the oxidation of nitrite (NO2−) by a hemoperoxidase.104 Nitropeptin (102) is a dipeptide antibiotic produced by Streptomyces xanthochromogenus 6257-MC1.105 The compound appears to be a mixture of diastereomers since the proton alpha to the nitro group undergoes exchange in D2O.105 Nitropeptin exhibits antibacterial activity against E. coli K-12 growing on synthetic medium, but the activity is reduced by supplementation with L-glutamine. Nitropeptin inhibits rice blast disease caused by the fungus Pyricularia oryzae.105The cyclic peptide antibiotics ilamycin (103), ilamycin B1 (104), and ilamycin B2 (105), which are produced by Streptomyces islandicus, each contain a 3-nitrotyrosine residue.106–109 Ilamycin B1 appears to be structurally identical to rufomycin B, which was isolated from Streptomyces atratus.110 Ilamycin (103) exhibits activity against human and bovine tubercular bacteria.111
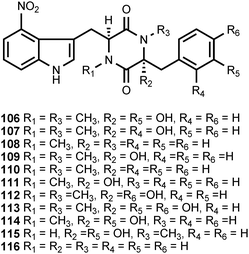
The thaxtomins (106–116) are a family of 2,5-diketopiperazines that contain a 4-nitrotryptophan residue. The thaxtomins are phytotoxins produced by Streptomyces scabies and at least five other Streptomyces species, including Streptomyces acidiscabies, Streptomyces turgidiscabies, Streptomyces europaeiscabiei, Streptomyces stelliscabiei, and Streptomyces niveiscabiei.112 These bacteria produce scab-like symptoms on several economically important root crops such as potato, beet, carrot, parsnip, radish, sweet potato, and turnip plants. The chemistry and biology of the thaxtomins have been reviewed by King and Calhoun.112 Investigations of the biosynthesis of the 4-nitrotryptophan residue present in these phytotoxins have revealed that a nitric oxide synthase (NOS) encoded in the thaxtomin biosynthetic gene cluster is required for the nitration reaction. The mechanism of the nitration is still somewhat unclear, although recent studies have established that 4-nitrotryptophan is an intermediate in thaxtomin biosynthesis.113
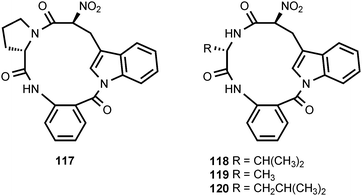
The nitrated peptide psychrophilin A (117) is produced by the psychrotolerant fungus Penicillium ribeum.114 The structurally related nitrated peptides psychrophilin B (118) and C (119) are produced by another psychrotolerant fungus, Penicillium rivulum.115 A fourth member of this family, psychrophilin D (120), is produced by Penicillium algidum.116 Psychrophilin D exhibits a moderate degree of anticancer activity, but no antibacterial, antimalarial, or antiviral activity.116
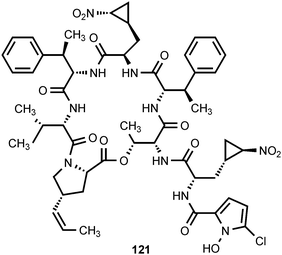
Hormaomycin (121) is a novel, cyclic peptide produced by Streptomyces griseoflavus.117 Its absolute configuration has been elucidated by Zlatopolskiy et al.118 Hormaomycin exhibits antibacterial activity against some Gram-positive bacteria.117 In addition, hormaomycin stimulates aerial mycelium formation and the production of other secondary metabolites. It therefore appears to act as an intercellular signaling molecule.117 Hormaomycin contains the unusual amino acid trans-3-(2-nitrocyclopropyl)alanine. Precursor incorporation experiments with S. griseoflavus have shown that this amino acid is derived from lysine via 4-hydroxylysine.119 The incorporation experiments suggest that the nitro group of trans-3-(2-nitrocyclopropyl)alanine is derived from the ε-amino group of lysine, although (ε-15N)-lysine was not evaluated as a precursor.119
5 Nitrated carbohydrates
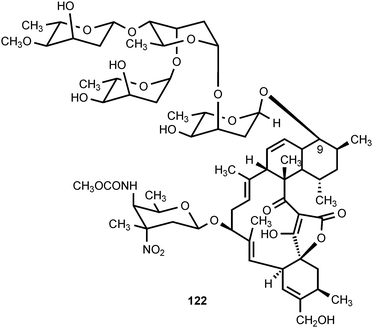
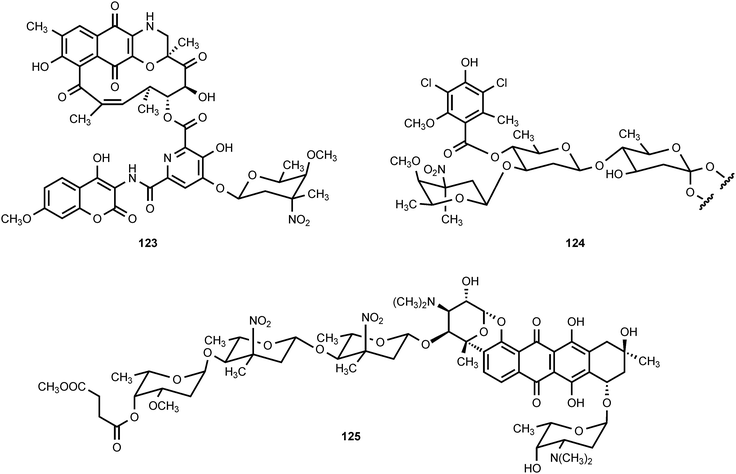
Four nitrated carbohydrates have been reported to occur in nature. D-Kijanose occurs in the spirotetronate, polyketide antibiotic kijanimicin (122), which is produced by Actinomadura kijaniata.120 It displays a broad spectrum of antimicrobial activity against Gram-positive bacteria, anaerobes, and the malaria parasite Plasmodium falciparum.121 Kijanimicin also shows antitumor activity.120,121D-Kijanose has also been found in the tetrocarcins,122,123 lobophorins,124 and arisostatins,125 all of which are spirotetronate natural products.A second nitrosugar, D-rubranitrose, is found in the antibiotic rubradirin (123), which is produced by Streptomyces achromogenes var. rubradiris.126–132 Rubradirin is highly active against Gram-positive bacteria and Haemophilus influenza.131 The third naturally occurring nitrosugar is L-evernitrose, which is found in everninomycin B and D (124, part structure) produced by Micromonospora carbonaceae.133–136 The everninomicins display potent activity against Gram-positive bacteria and multiply-drug-resistant Neisseria.135 The fourth nitrosugar is L-decilonitrose, which is found in the polyketide antibiotic decilorubicin (125) produced by Streptomyces virginiae,137–141 and in the related antibiotics arugomycin,142–147 cororubicin,148 and viriplanin A.149,150 Decilorubicin exhibits activity against Gram-positive bacteria as well as antitumor activity.137
Insight into the biosynthesis of these nitro sugars was first obtained by the isolation of protorubradirin from Streptomyces achromogenes var. rubradiris.130 Protorubradirin is structurally identical to rubradirin except for the presence a C-nitroso sugar. On exposure to light and air, protorubridirin is converted into rubradirin suggesting that the latter compound is an artifact.130 More recently, genomic analysis and experiments with overproduced proteins have shown that both the rubradirin and everninomicin biosynthetic gene clusters132,151 encode flavin monooxygenases that can oxidize the amino group of the substrate analog L-TDP-3-epi-vancosamine to the corresponding nitroso TDP-sugar derivative.152 The reaction appears to involve the sequential oxidation of the amino group to a hydroxylamine followed by oxidation of the hydroxylamine moiety to a nitroso group.
6 Nitrated aliphatic compounds
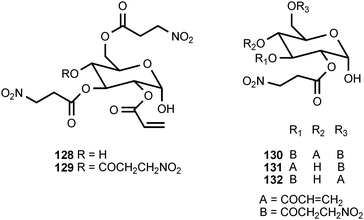
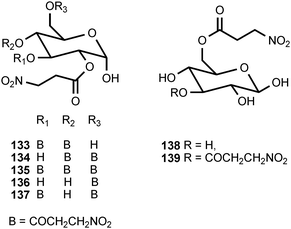
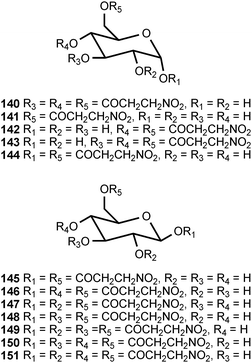
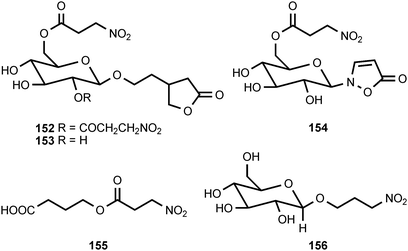
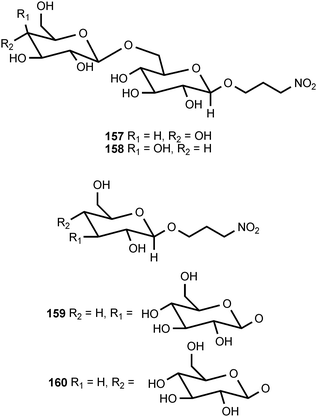
Of all the naturally occurring nitrated compounds, 3-nitropropanoic acid (126) exhibits some of the most profound effects on humans and domestic livestock.153 The widespread distribution of 3-nitropropanoic acid in range plants and the deadly effects of ingestion combine to make the compound of concern in agriculture worldwide. “Moldy sugarcane toxicity” is the most common cause of human deaths from 3-nitropropanoic acid ingestion.153 3-Nitropropanoic acid and its derivatives are produced by legumes in the genera Astragalus, Coronilla, Indigofera, Lotus, Hippocrepis, and Scorpiurus. The compound is also synthesized by certain plant species in the Malpighiaceae, Corynocarpaceae, and Violaceae.153,154 In addition, free 3-nitropropanoate is produced by fungal species in the genera Aspergillus, Penicillium, and Arthrinium,153,154 and more recently has been isolated from endophytic fungi.155 In vascular plants, 3-nitropropanoic acid occurs both in free form and as glucose esters and glycosides.154 Plants are believed to produce 3-nitropropanoic acid and its derivatives as a deterrent to herbivory. In ruminants, the glucose conjugates are rapidly hydrolyzed in the rumen to liberate free 3-nitropropanoate.154 Ingestion of plants containing these compounds by livestock leads to a variety of pathological symptoms including incontinence, loss of hindquarter coordination, and excessive salivation.154 The biological effects of 3-nitropropanoate result from its action as a suicide inactivator of the enzyme succinate dehydrogenase.156,157 3-Nitropropanol (127) occurs with 3-nitropropanoate in free and bound form in Astragalus spp.158,159 3-Nitropropanol is not toxic to mammals, but it is converted to 3-nitropropanoate by hepatic liver alcohol dehydrogenase.154A variety of glucose esters and glycosides of 3-nitropropanoate have been isolated. The mixed 3-nitropropanoyl acryloyl esters 128–132 are found in Indigofera kirilowii and Indigofera carlesii.160–162 The 3-nitropropanoate glucose esters and glucosides 133–151 have been found in species of Astragalus,159,163Hiptage,164Corynocarpus,165–167Indigofera,160–162,168–170 and Coronilla.171,172 The two esters 152 and 153, which contain a 5-oxotetrahydrofuran-3-acetic acid residue, occur in the Canada milk-vetch, Astragalus canadensis.163 The isoxazolinone derivative 154 was first isolated as a major component of the defense glands of chrysomelid beetles,173 and it was subsequently found in A. canadensis and Astragalus collinus.174 The 3-nitropropanoyl ester of 4-hydroxybutanoic acid (155) occurs in Hippocrepis comosa.175 The 3-nitropropanol glucoside miserotoxin (156), which was first isolated from Astragalus miser var. oblongifolius, occurs in at least nine other species of Astragalus.159,176 In the timber milk-vetch, A. miser var. serotinus, miserotoxin is accompanied by the disaccharides 157–160.177–180
The biosynthesis of 3-nitropropanoate appears to be significantly different in fungi and higher plants. In Penicillium atrovenetum, 3-nitropropanoate is derived from L-aspartate by oxidation to produce L-nitrosuccinate, which then decarboxylates to yield 3-nitropropanoate.181 Investigations of 3-nitropropanoate biosynthesis in Indigofera spicata (creeping indigo) demonstrated that radiolabeled aspartic acid was not incorporated into 3-nitropropanoate. Rather, evidence supporting a role for malonic acid and malonyl monohydroxamate in 3-nitropropanoate biosynthesis was obtained.182
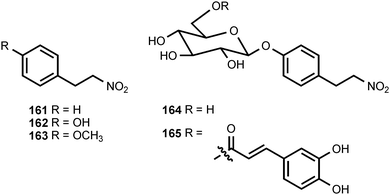
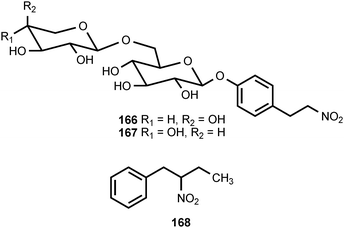
1-Nitro-2-phenylethane (161) and (2-nitrobutyl)benzene (168) have been isolated from the volatile flavor components of the African Mobola plum Parinari curatellifolia (Chrysobalanaceae),183 and 161 has been identified as an aroma component of tomatoes.184 1-Nitro-2-phenylethane has also been reported to be produced from tyrosine by a microsomal system from Sorghum bicolor (Poaceae)185 and by cell suspension cultures of Eschscholtzia californica (Papaveraceae).186 In Eschscholtzia, both molecular oxygen and NADPH are required for the conversion of tyrosine into 161. The related compounds 1-nitro-2-(4-hydroxyphenyl)ethane (162) and 1-nitro-2-(4-methoxyphenyl)ethane (163) are produced as stress metabolites in leaves of the Western skunk cabbage, Lysichitum americanum (Araceae), treated with cupric chloride.187 The former compound exhibited antifungal activities against Fusarium oxysporum and Cladosporium herbarum.187 A glycoside of 1-nitro-2-(4-hydroxyphenyl)ethane called thalictoside (164) has been isolated from Thalictrum aquilegifolium (Ranunculaceae).188 The related caffeoyl ester 165 occurs in the bark of Parabenzoin praecox (Lauraceae).189 The tubers of Sagittaria trifolia (Alismataceae), which are used in traditional Chinese medicine, contain the related disaccharide arabinothalictoside (166).190 The epimeric disaccharide 167 occurs in Diploclisia glaucescens (Menispermaceae)191 and the roots of another traditional Chinese medicine, Semiaquilegia adoxoides (Ranunculaceae).192
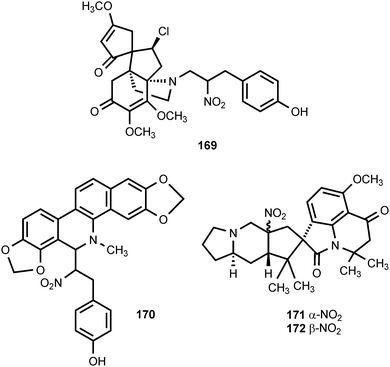
The rhizomes of the traditional Chinese medicinal plant Menispermum dauricum (Menispermaceae) contain the novel alkaloid nitrotyrasacutiminine (169).193 This plant is used as an analgesic and antipyretic.193 The unusual benzophenanthridine alkaloid nitrotyrasanguinarine (170) is produced by Hypecoum imberbe, Hypecoum procumbens, and Hypecoum pendulum (Hypecoaceae).194 The authors speculate that the biosynthesis of 170 begins with the oxidation of tyramine to 2-(4-hydroxyphenyl)-1-nitroethane. Penicillium cyclopium is a frequent contaminant of stored grains and cereals. Cyclopiamines A (171) and B (172) are alkaloids isolated from a toxigenic strain of this organism.195 The structures of these alkaloids are similar to cyclopiazonic acid, the major toxin produced by the same fungus.196
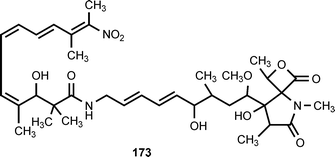
The novel antibiotic lajollamycin (173) is produced by a strain of Streptomyces nodosus isolated from marine sediment collected in Scripps Canyon in La Jolla, California. Lajollamycin exhibits antimicrobial activity against methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus faecalis (VRE). It also inhibits the growth of murine melanoma cell line B16-F10.197
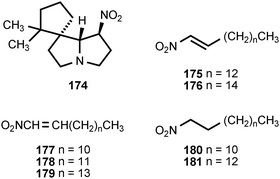
Several arthropods engage in chemical warfare with the assistance of nitrated compounds. The spirocyclic alkaloid nitropolyzonamine (174) has been isolated from the defense glands of the millipede Polyzonium rosalbum.198,199 The compound, secreted in response to ant attack, causes the ants to disperse and engage in cleaning behavior.200 Two of the seven termite families use chemical defense, and at least three species of rhinotermitids, utilize nitroalkenes as an interspecies contact poison.201 The frontal glands of soldiers of three Prorhinotermes sp., Prorhinotermes canalifrons, Prorhinotermes inopinatus, and Prorhinotermes simplex contain nitroalkenes and nitroalkanes. The major component of the glands is (E)-1-nitropentadec-1-ene (175). Other components include the nitroalkenes 176–179 and the nitroalkanes 180 and 181.202 The mechanism for the formation of the nitro group in these compounds is unknown.
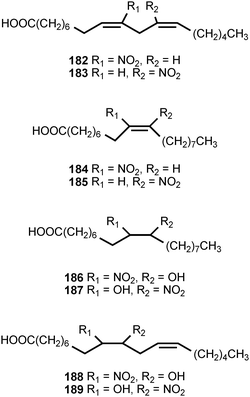
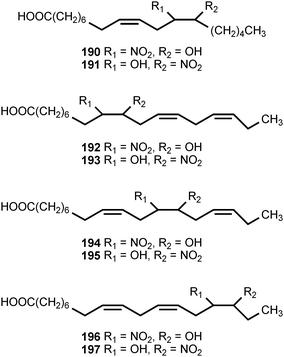
Mass-spectrometric analysis of human urine and plasma has revealed the presence of nitrated derivatives of all the major unsaturated fatty acids.203,204 The compounds detected include the nitroalkenes and hydroxynitroalkanes 182–197. Formation of the nitrated fatty acid derivatives is ascribed to nitration by the cascade of reactive nitrogen species derived from nitric oxide. The hydroxynitroalkanes appear to result from the Michael addition of water to the corresponding nitroalkenes. The unsaturated nitroalkenes 182 and 183 derived from linoleic acid are present in healthy human red blood cells at concentrations of ca. 500 nM, whereas the nitroalkenes 184 and 185 derived from oleic acid are present at ca. 50% greater concentrations. The combined concentrations of free and esterified nitrolinoleic and nitrooleic acids exceed 1 μM.203 The nitrated forms of linoleic and oleic acids exhibit strong cell signaling properties resulting from activation of peroxisome proliferators-activated receptors (PPARs), a class of nuclear hormone receptors that modulates expression of genes causing cellular differentiation and inflammation. The nitrated oleic acids are more potent than the nitrated linoleic acids in the activation of the γ-PPAR receptor. The hydroxynitroalkane derivatives 186–197 do not serve as ligands for the γ-PPAR receptor.203
7 O-Nitro and N-nitro compounds
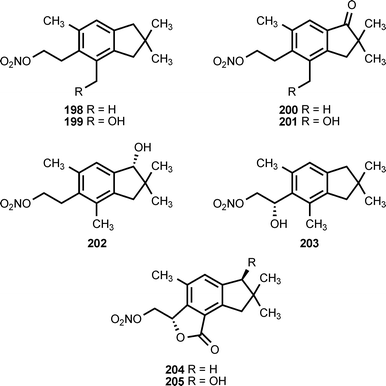
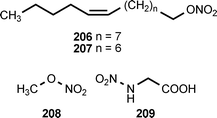
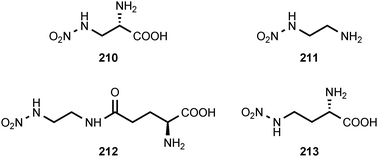
Natural products containing O-nitro or N-nitro groups are relatively rare, and no information on their biosynthesis is currently available. The sub-Antarctic soft coral Alcyonium paessleri contains an assortment of illudalane sesquiterpenes. Eight of these compounds, alcyopterosins B (198), C (200), E (204), F (203), G (199), H (202), J (201) and M (205), contain a nitrate ester moiety. Alcyopterosin E shows mild cytotoxicity toward a human larynx carcinoma cell line, while alcyopterosins C and H are cytotoxic toward a human colon carcinoma cell line at 10 μg mL−1.205 (Z)-9-Tetradecenyl nitrate (206) and (Z)-8-tridecenyl nitrate (207) have been identified as components of the sex pheromone produced by virgin female cotton leaf perforator moths, Bucculatrix thurberiella.206 The presence of methyl nitrate (208) in exhaled human breath has been identified as a marker for type 1 diabetes mellitus in children.207N-Nitroglycine (209) has been extracted from the culture broth of Streptomyces noursei. The compound exhibits weak antibacterial activity.208L-2-Nitraminoalanine (210) and its decarboxylation product 1-amino-2-nitraminoethane (211) have been found in the fruiting bodies of Agaricus sylvaticus.209 The N-nitro compounds 210, 211, and 212 occur in the fruiting bodies of Agaricus subrutilescens, along with L-4-nitramino-2-aminobutanoic acid (213).210 Compounds 210 and 213 were assigned the L-configuration on the basis of their optical rotations.210 The biological activities of these compounds have not been reported.8 Conclusion
It is clear from the above survey that nitro-substituted organic compounds are ubiquitous in the biosphere. The biosynthesis, biodegradation, and ecological functions of most of these compounds are largely uninvestigated. It seems likely that the molecules discovered to date represent only a fraction of the diversity of nitrated natural products. Further explorations of that diversity along with investigations of the biochemistry and biology associated with these substances will provide a fruitful area for additional research that should lead to many pharmaceutical and agricultural applications.
Note added in proof
The X-ray structure of KijD3, a key enzyme in the biosynthesis of D-kijanose, has been determined.211 KijD3 is an FAD-dependent oxidoreductase that catalyzes the oxidation of TDP-3-amino-2,3,6-trideoxy-4-keto-3-methyl-D-glucose to the corresponding hydroxyamino sugar.211 The X-ray structure and mechanism of ORF36, an amino sugar oxidizing enzyme involved in everninomicin biosynthesis, have also been elucidated.212 ORF36 is an FAD-dependent oxidoreductase that catalyzes the oxidation of TDP-L-epi-vancosamine to the corresponding nitroso sugar derivative.212 The biosynthesis of hydroxyaminosugars, nitrososugars, and nitrosugars has been reviewed.213
The production of pyrrolnitrin in several Burkholderia species has been shown to be regulated by N-acylhomoserine lactone (AHL)-dependent quorum sensing.214
9 Acknowledgements
The authors thank the Office of Naval Research (N00014-08-1-0472 to R.J.P), the Robert A. Welch Foundation (C-0729 to R.J.P), and the Defense Threat Reduction Agency and the Army Research Office (W911NF-07-1-0077 to J.C.S. and S.F.N.) for grant support. We thank Se Na Yoo for assistance with compiling the structures.10 References
- R. Winkler and C. Hertweck, ChemBioChem, 2007, 8, 973–977 CrossRef CAS.
- K. S. Ju and R. E. Parales, Microbiol. Mol. Biol. Rev., 2010, 74, 250–272 CrossRef CAS.
- W. Al-Zereini, I. Schuhmann, H. Laatsch, E. Helmke and H. Anke, J. Antibiot., 2007, 60, 301–308 CrossRef CAS.
- I. Schuhmann, C. B. F.-F. Yao, W. Al-Zereini, H. Anke, E. Helmke and H. Laatsch, J. Antibiot., 2009, 62, 453–460 CrossRef CAS.
- S. I. Sviridov and B. S. Ermolinskii, Chem. Nat. Compd., 1990, 26, 691–696 CrossRef.
- M. Tischler, S. W. Ayer and R. J. Andersen, Comp. Biochem. Physiol., 1986, 84B, 43–46 CAS.
- X. Fu, F. J. Schmitz and R. S. Tanner, J. Nat. Prod., 1995, 58, 1950–1954 CrossRef CAS.
- G. Neme, M. Nieto, A. T. D'Arcangelo and E. G. Gros, Phytochemistry, 1977, 16, 277–278 CrossRef CAS.
- B. Dinda, U. C. De, B. Achari, S. Arima, N. Sato and Y. Harigaya, Ind. J. Chem., Sect. B, 2003, 42, 1514–1518.
- R. B. Herbert and A. R. Knaggs, Tetrahedron Lett., 1988, 29, 6353–6356 CrossRef CAS.
- V. Thaller and J. L. Turner, J. Chem. Soc., Perkin Trans. 1, 1972, 2032–2034 RSC.
- J. He and C. Hertweck, J. Am. Chem. Soc., 2004, 126, 3694–3695 CrossRef CAS.
- M. Zikmundová, K. Drandarov, L. Bigler, M. Hesse and C. Werner, Appl. Environ. Microbiol., 2002, 68, 4863–4870 CrossRef CAS.
- R. R. King, C. H. Lawrence and L. A. Calhoun, Phytochemistry, 1998, 49, 1265–1267 CrossRef CAS.
- D. Butruille and X. A. Dominguez, Tetrahedron Lett., 1972, 13, 211–212 CrossRef.
- G. Höfle and H. Irschik, J. Nat. Prod., 2008, 71, 1946–1948 CrossRef.
- C.-L. Shao, Z.-Y. Guo, X.-K. Xia, Y. Liu, Z.-J. Huang, Z.-G. She, Y.-C. Lin and S.-N. Zhou, J. Asian Nat. Prod. Res., 2007, 9, 643–648 CrossRef CAS.
- T. D. Brock, Bacteriol. Rev, 1961, 25, 32–48 CAS.
- L. C. Vining and C. Stuttard, in Genetics and Biochemistry of Antibiotic Production, ed. L. C. Vining and C. Stuttard, Butterworth-Heinemann, Boston, 1995, pp. 505–530 Search PubMed.
- M. A. Xaplanteri, A. Andreou, G. P. Dinos and D. L. Kalpaxis, Nucleic Acids Res., 2003, 31, 5074–5083 CrossRef CAS.
- K. Shirahata, T. Hayashi, T. Deguchi, T. Suzuki and I. Matsubara, Agric. Biol. Chem., 1972, 36, 2229–2232 CAS.
- T. Suzuki, H. Honda and R. Katsumata, Agric. Biol. Chem., 1972, 36, 2223–2238 CAS.
- E. A. Lewis, T. L. Adamek, L. C. Vining and R. L. White, J. Nat. Prod., 2003, 66, 62–66 CrossRef CAS.
- M. M. A. El-Gendy, U. W. Hawas and M. Jaspars, J. Antibiot., 2008, 61, 379–386 CrossRef CAS.
- M. Lang, P. Spiteller, V. Hellwig and W. Steglich, Angew. Chem., Int. Ed., 2001, 40, 1704–1705 CrossRef CAS.
- T. Shomura, S. Amano, J. Yoshida, N. Ezaki, T. Ito and T. Niida, Int. J. Syst. Bacteriol., 1983, 33, 557–564 Search PubMed.
- N. Ezaki, T. Shomura, M. Koyama, T. Niwa, M. Kojima, S. Inouye, T. Ito and T. Niida, J. Antibiot., 1981, 34, 1363–1365 CAS.
- K. Maeda, T. Osato and H. Umezawa, J. Antibiot., 1953, 6, 182 CAS.
- Y. Okami, K. Maeda and H. Umezawa, J. Antibiot., 1954, 7, 53–56 CAS.
- T. Osato, M. Ueda, S. Fukuyama, K. Yagishita, Y. Okami and H. Umezawa, J. Antibiot., 1955, 8, 105–109 CAS.
- J. I. Shoji, H. Hinoo, Y. Terui, J. Kikuchi, T. Hattori, K. Ishii, K. Matsumoto and T. Yoshida, J. Antibiot., 1989, 42, 1513–1514 CAS.
- P. Joseph, A. K. Jaiswal, C. C. Stobbe and J. D. Chapmaan, Int. J. Radiat. Oncol., Biol., Phys., 1994, 29, 351–355 CrossRef CAS.
- F. Shintani, T. Kinoshita, S. Kanba, T. Ishikawa, E. Suzuki, N. Sasakawa, R. Kato, M. Asai and T. Nakaki, J. Biol. Chem., 1996, 271, 13561–13565 CrossRef CAS.
- A. Chiari, X.-H. Li, Z. Xu, H.-L. Pan and J. C. Eisenach, Neuroscience, 2000, 101, 189–196 CrossRef CAS.
- W. Fenical and P. R. Jensen, Nat. Chem. Biol., 2006, 2, 666–673 CrossRef CAS.
- T. Minoda, T. Kodama, T. Omori and S. Hagihara, Japan Kokai Tokkyo Koho, JP 53133689, Japan, 1978 Search PubMed.
- T. Ohmori, S.-I. Hagiwara, A. Ueda, Y. Minoda and K. Yamada, Agric. Biol. Chem., 1978, 42, 2031–2036 CAS.
- N. Ezaki, M. Koyama, T. Shomura, T. Tsuruoka and S. Inouye, J. Antibiot., 1983, 36, 1263–1267 CAS.
- M. Koyama, N. Ezaki, T. Tsuruoka and S. Inouye, J. Antibiot., 1983, 36, 1483–1489 CAS.
- H. Nakamura, K. Shiomi, H. Iinuma, H. Naganawa, T. Obata, T. Takeuchi, H. Umezawa, Y. Takeuchi and Y. Iitaka, J. Antibiot., 1987, 40, 899–903 CAS.
- K. Masuda, K. Suzuki, A. Ishida-Okawara, S. Mizuno, K. Hotta, S. Miyadoh, O. Hara and M. Koyama, J. Antibiot., 1991, 44, 533–540 CAS.
- R. D. Charan, G. Schlingmann, V. S. Bernan, X. Feng and G. T. Carter, J. Nat. Prod., 2005, 68, 277–279 CrossRef CAS.
- X. Zhang and R. J. Parry, Antimicrob. Agents Chemother., 2007, 51, 946–957 CrossRef CAS.
- A. S. Ratnayake, B. Haltli, X. Feng, V. S. Bernan, M. P. Singh, H. He and G. T. Carter, J. Nat. Prod., 2008, 71, 1923–1926 CrossRef CAS.
- G. T. Carter, J. A. Nietsche, J. J. Goodman, M. J. Torrey, T. S. Dunne, M. M. Siegel and D. B. Borders, J. Chem. Soc., Chem. Commun., 1989, 1271–1273 RSC.
- K. Arima, H. Imanaka, M. Kousaka, A. Fukuda and G. Tamura, J. Antibiot., 1965, 18, 201–204 CAS.
- R. Hamill, R. Elander, J. Mabe and M. Gorman, Antimicrob. Agents Chemother., 1967, 7, 388–396 CAS.
- K. Gerth, W. Trowitzsch, V. Wray, G. Hofle, H. Irschik and H. Reichenbach, J. Antibiot., 1982, 35, 1101–1103 CAS.
- L. Chernin, A. Brandis, Z. Ismailov and I. Chet, Curr. Microbiol., 1996, 32, 208–212 CrossRef CAS.
- M. Hashimoto and K. Hattori, Chem. Pharm. Bull., 1966, 14, 1314–1316 CAS.
- M. Hashimoto and K. Hattori, Bull. Chem. Soc. Jpn., 1966, 39, 410 CrossRef CAS.
- J. N. Roitman, N. E. Mahoney and W. J. Janisiewicz, Appl. Microbiol. Biotechnol., 1990, 34, 381–386 CAS.
- N. El-Banna and G. Winkelmann, J. Appl. Microbiol., 1998, 85, 69–78 CrossRef CAS.
- P. E. Hammer, D. S. Hill, S. T. Lam, K.-H. Van Pée and J. M. Ligon, Appl. Environ. Microbiol, 1997, 63, 2147–2154 CAS.
- S. Kirner, P. E. Hammer, D. S. Hill, A. Altmann, I. Fischer, L. J. Weislo, M. Lanahan, K.-H. van Pée and J. M. Ligon, J. Bacteriol., 1998, 180, 1939–1943 CAS.
- J. Lee, M. Simurdiak and H. Zhao, J. Biol. Chem., 2005, 280, 36719–36727 CrossRef CAS.
- J. Lee and H. Zhao, Angew. Chem., Int. Ed., 2006, 45, 622–625 CrossRef CAS.
- M. Z. Sultan, K. Park, S. Y. Lee, J. K. Park, T. Varughese and S.-S. Moon, J. Antibiot., 2008, 61, 420–425 CrossRef CAS.
- Y. Hirata, H. Nakata, K. Yamada, K. Okuhara and T. Naito, Tetrahedron, 1961, 14, 252–274 CrossRef CAS.
- Y. Ishibashi, S. Ohba, S. Nishiyama and S. Yamamura, Bull. Chem. Soc. Jpn., 1995, 68, 3643–3649 CrossRef CAS.
- J.-Y. Ueda, J. Hashimoto, A. Nagai, T. Nakashima, H. Komaki, K. Anzai, S. Harayama, T. Doi, T. Takahashi, K. Nagasawa, T. Natsume, M. Takagi and K. Shin-ya, J. Antibiot., 2007, 60, 321–324 CrossRef CAS.
- C. Cardani, D. Ghiringhelli, A. Selva, F. Arcamone, B. Camerino and G. Cassinelli, Chim. Ind., 1970, 52, 793–794 Search PubMed.
- K. Kakinuma, C. A. Hanson and K. L. Rinehart Jr, Tetrahedron, 1976, 32, 217–222 CrossRef CAS.
- Y. Ishibashi, S. Nishiyama and S. Yamamura, Chem. Lett., 1994, 23, 1747–1748 CrossRef.
- Y. Koyama, Y. Fukakusa, N. Kyomura and S. Yamagishi, Tetrahedron Lett., 1969, 10, 355–358 CrossRef.
- M. G. Nair, A. Chandra, D. L. Thorogod and R. M. G. Davis, Pest. Sci., 1995, 43, 361–365 CAS.
- M. G. Nair, A. Chandra and D. L. Thorogood, J. Antibiot., 1993, 46, 1762–1763 CAS.
- S. Yamagishi, Y. Koyama, Y. Fukakusa, N. Kyomura, J.-I. Ohishi, N. Hamamachi and T. Arai, Yakugaku Zasshi, 1971, 91, 351–357 CAS.
- J. He and C. Hertweck, Chem. Biol., 2003, 10, 1225–1232 CrossRef CAS.
- J. He and C. Hertweck, ChemBioChem, 2005, 6, 908–912 CrossRef CAS.
- N. Traitcheva, H. Jenke-Kodama, J. He, E. Dittmann and C. Hertweck, ChemBioChem, 2007, 8, 1841–1849 CrossRef CAS.
- R. Winkler and C. Hertweck, Angew. Chem., Int. Ed., 2005, 44, 4083–4087 CrossRef CAS.
- M. Simurdiak, J. Lee and H. Zhao, ChemBioChem, 2006, 7, 1169–1172 CrossRef CAS.
- R. Winkler, M. E. A. Richter, U. Knupfer and C. Hertweck, Angew. Chem., Int. Ed., 2006, 45, 8016–8018 CrossRef CAS.
- R. Winkler, G. Zocher, I. Richter, T. Friedrich, G. E. Schulz and C. Hertweck, Angew. Chem., Int. Ed., 2007, 46, 8605–8608 CrossRef CAS.
- C. Krebs, M. L. Matthews, W. Jiang and J. M. BollingerJr, Biochemistry, 2007, 46, 10413–10418 CrossRef CAS.
- Y. S. Choi, H. Zhang, J. S. Brunzelle, S. K. Nair and H. Zhao, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 6858–6863 CrossRef CAS.
- V. K. Korboukh, N. Li, E. W. Barr, J. M. Bollinger Jr and C. Krebs, J. Am. Chem. Soc., 2009, 131, 13608–13609 CrossRef CAS.
- N. Li, V. K. Korboukh, C. Krebs and J. M. BollingerJr, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 15722–15727 CrossRef CAS.
- K. Kurosawa, K. Takahashi and E. Tsuda, J. Antibiot., 2001, 54, 541–547 CAS.
- K. Takahashi, E. Tsuda and K. Kurosawa, J. Antibiot., 2001, 54, 548–553 CAS.
- M. Muller, B. Kusebauch, G. Liang, C. M. Beaudry, D. Trauner and C. Hertweck, Angew. Chem., Int. Ed., 2006, 45, 7835–7838 CrossRef CAS.
- L.-Y. Tao, J.-Y. Zhang, Y.-J. Liang, L.-M. Chen, L.-S. Zheng, F. Wang, Y.-J. Mi, Z.-G. She, K. K. W. To, Y.-C. Lin and L.-W. Fu, Mar. Drugs, 2010, 8, 1094–1105 CrossRef CAS.
- A. A. Tymiak, C. A. Culver, M. F. Malley and J. Z. Gougoutas, J. Org. Chem., 1985, 50, 5491–5495 CrossRef CAS.
- J. S. Wells, W. H. Trejo, P. A. Principe and R. B. Sykes, J. Antibiot., 1984, 37, 802–803 CAS.
- K. Krohn, S. F. Kouam, S. Cludius-Brandt, S. Draeger and B. Schulz, Eur. J. Org. Chem., 2008, 2008, 3615–3618 CrossRef.
- V. Kumar, Poonam, A. K. Prasad and V. S. Parmar, Nat. Prod. Rep., 2003, 20, 565–583 RSC.
- V. M. Arlt, M. Stiborova and H. H. Schmeiser, Mutagenesis, 2002, 17, 265–277 CrossRef CAS.
- H. Dong, N. Suzuki, M. C. Torres, R. R. Bonala, F. Johnson, A. P. Grollman and S. Shibutani, Drug Metab. Dispos., 2006, 34, 1122–1127 CrossRef CAS.
- F. Comer, H. P. Tiwari and I. D. Spenser, Can. J. Chem., 1969, 47, 481–487 CrossRef CAS.
- H. Schutte, U. Orban and K. Mothes, Eur. J. Biochem., 1967, 1, 70–72 CrossRef CAS.
- V. Sharma, S. Jain, D. S. Bhakuni and R. S. Kapil, J. Chem. Soc., Perkin Trans. 1, 1982, 1153–1155 RSC.
- Y. R. Zhao, T. F. Zhao, S. Lai and X. K. Wang, Chin. Chem. Lett., 1993, 4, 145–148 CAS.
- X.-K. Wang, Y.-R. Zhao, T.-F. Zhao, S. Lai and C. Chun-tao, Phytochemistry, 1993, 35, 263–265 CrossRef.
- H. Zhang, F.-D. Wang and J.-M. Yue, Chin. J. Chem., 2006, 24, 781–784 CrossRef CAS.
- C. A. Carollo and J. M. de Siqueira, Nat. Prod. Res., 2009, 23, 633–637 CrossRef CAS.
- H. L. Li, W. D. Zhang, W. Zhang, C. Zhang and R. H. Liu, Chin. Chem. Lett., 2005, 16, 367–368 CAS.
- S. W. Ayer, R. J. Andersen, C.-H. He and J. Clardy, J. Org. Chem., 1984, 49, 3869–3870 CrossRef CAS.
- X. Wang, Z. Li and B. Yang, Fitoterapia, 2004, 75, 789–791 CrossRef CAS.
- H. B. Park, H. C. Kwon, C.-H. Lee and H. O. Yang, J. Nat. Prod., 2009, 72, 248–252 CrossRef CAS.
- B. F. Burrows and W. B. Turner, J. Chem. Soc. (C), 1966, 255–260 RSC.
- D. Broadbent and M. E. Radley, Ann. Bot., 1966, 30, 763–777 CAS.
- S. A. B. Greenacre and H. Ischiropoulos, Free Radical Res., 2001, 34, 541–581 CrossRef CAS.
- J. M. Souza, G. Peluffo and R. Radi, Free Radical Biol. Med., 2008, 45, 357–366 CrossRef CAS.
- K. Ohba, H. Nakayama, K. Furihata, A. Shimazu, T. Endo, H. Seto and N. Otake, J. Antibiot., 1987, 40, 709–713 CAS.
- T. Takita, K. Oi, Y. Okami, K. Maeda and H. Umezawa, J. Antibiot., 1962, 15, 46–48 CAS.
- T. Takita, H. Naganawa, K. Maeda and H. Umezawa, J. Antibiot., 1964, 17, 264–265 CAS.
- T. Takita, H. Naganawa, K. Maeda and H. Umezawa, J. Antibiot., 1964, 17, 90–91 CAS.
- T. Takita, H. Naganawa, K. Maeda and H. Umezawa, J. Antibiot., 1964, 17, 129–131 CAS.
- M. Fujino, T. Kamiya, H. Iwasaki, J. Ueyanagi and A. Miyake, Chem. Pharm. Bull., 1964, 12, 1390–1392 CAS.
- R. Kaku, J. Antibiot., 1963, 16, 93–98 CAS.
- R. R. King and L. A. Calhoun, Phytochemistry, 2009, 70, 833–841 CrossRef CAS.
- E. G. Johnson, S. B. Krasnoff, D. R. D. Bignell, W.-C. Chung, T. Tao, R. J. Parry, R. Loria and D. M. Gibson, Mol. Microbiol., 2009, 73, 409–418 CrossRef CAS.
- P. W. Dalsgaard, T. O. Larsen, K. Frydenvang and C. Christophersen, J. Nat. Prod., 2004, 67, 878–881 CrossRef CAS.
- P. W. Dalsgaard, J. W. Blunt, M. H. G. Munro, T. O. Larsen and C. Christophersen, J. Nat. Prod., 2004, 67, 1950–1952 CrossRef CAS.
- P. W. Dalsgaard, T. O. Larsen and C. Christophersen, J. Antibiot., 2005, 58, 141–144 CrossRef CAS.
- E. Rössner, A. Zeeck and W. A. König, Angew. Chem., Int. Ed. Engl., 1990, 29, 64–65 CrossRef.
- B. D. Zlatopolskiy, K. Loscha, P. Alvermann, S. I. Kozhushkov, S. V. Nikolaev, A. Zeeck and A. de Meijere, Chem.–Eur. J., 2004, 10, 4708–4717 CrossRef CAS.
- M. Brandl, S. I. Kozhushkov, B. D. Zlatopolskiy, P. Alvermann, B. Geers, A. Zeeck and A. de Meijere, Eur. J. Org. Chem., 2005, 123–135 CrossRef CAS.
- A. K. Mallams, M. S. Puar, R. R. Rossman, A. T. McPhail, R. D. Macfarlane and R. L. Stephens, J. Chem. Soc., Perkin Trans. 1, 1983, 1497–1534 RSC.
- H. Zhang, J. A. White-Phillip, C. E. Melancon 3rd, H. J. Kwon, W. L. Yu and H. W. Liu, J. Am. Chem. Soc., 2007, 129, 14670–14683 CrossRef CAS.
- T. Tamaoki, M. Kasai, K. Shirahata, S. Ohkubo, M. Morimoto, K. Mineura, S. Ishii and F. Tomita, J. Antibiot., 1980, 33, 946–950 CAS.
- T. Tamaoki, M. Kasai, K. Shirahata and F. Tomita, J. Antibiot., 1982, 35, 979–984 CAS.
- Z. D. Jiang, P. R. Jensen and W. Fenical, Bioorg. Med. Chem. Lett., 1999, 9, 2003–2006 CrossRef CAS.
- Y. Igarashi, K. Takagi, Y. Kan, K. Fujii, K.-I. Harada, T. Furumai and T. Oki, J. Antibiot., 2000, 53, 233–240 CAS.
- H. Hoeksema, S. A. Mizsak and L. Baczynskyj, J. Antibiot., 1979, 32, 773–776 CAS.
- S. A. Mizsak, H. Hoeksema and L. M. Pschigoda, J. Antibiot., 1979, 32, 771–772 CAS.
- H. Hoeksema, C. Lewis, S. A. Mizsak, J. A. Shiley, D. R. Wait, H. A. Whaley and G. E. Zurenko, J. Antibiot., 1978, 31, 945–948 CAS.
- H. Hoeksema, C. Chidester, S. A. Mizsak and L. Baczynskyj, J. Antibiot., 1978, 31, 1067–1069 CAS.
- B. Bannister and B. A. Zapotocky, J. Antibiot., 1992, 45, 1313–1324 CAS.
- H. Hoeksema, S. A. Mizak, L. Baczynskyj and L. M. Pschigoda, J. Am. Chem. Soc., 1982, 104, 5173–5181 CrossRef CAS.
- C. G. Kim, J. Lamichhane, K. I. Song, V. D. Nguyen, D. H. Kim, T. S. Jeong, S. H. Kang, K. W. Kim, J. Maharjan, Y. S. Hong, J. S. Kang, J. C. Yoo, J. J. Lee, T. J. Oh, K. Liou and J. K. Sohng, Arch. Microbiol., 2008, 189, 463–473 CrossRef CAS.
- A. K. Ganguly, O. Z. Sarre and H. Reimann, J. Am. Chem. Soc., 1968, 90, 7129–7130 CrossRef CAS.
- M. J. Weinstein, G. M. Luedemann, E. M. Oden and G. H. Wagman, Antimicrob. Agents Chemother., 1965, 24–32 CAS.
- A. K. Ganguly, O. Z. Sarre, D. Greeves and J. Morton, J. Am. Chem. Soc., 1975, 97, 1982–1985 CrossRef CAS.
- A. K. Ganguly, O. Z. Sarre, A. T. McPhail and K. D. Onan, Chem. Commun., 1977, 9, 313–314 Search PubMed.
- K. Ishii, S. Kondo, Y. Nishimura, M. Hamada, T. Takeuchi and H. Umezawa, J. Antibiot., 1983, 36, 451–453 CAS.
- K. Ishii, Y. Nishimura, S. Kondo and H. Umezawa, J. Antibiot., 1983, 36, 454–456 CAS.
- K. Ishii, Y. Nishimura, H. Naganawa, S. Kondo and H. Umezawa, J. Antibiot., 1984, 37, 344–353 CAS.
- Y. Nishimura, K. Ishii and S. Kondo, J. Antibiot., 1990, 43, 54–61 CAS.
- P. Deshong, W. Li, J. W. J. Kennington and H. L. Ammon, J. Org. Chem., 1991, 56, 1364–1373 CrossRef CAS.
- H. Kawai, Y. Hayakawa, M. Nakagawa, K. Furihata, H. Seto and N. Otake, Tetrahedron Lett., 1984, 25, 1941–1944 CrossRef CAS.
- H. Kawai, Y. Hayakawa, M. Nakagawa, K. Furihata, H. Seto and N. Otake, Tetrahedron Lett., 1984, 25, 1937–1940 CrossRef.
- H. Kawai, Y. Hayakawa, M. Nakagawa, K. Imamura, K. Tanabe, A. Shimazu, H. Seto and N. Ōtake, J. Antibiot., 1983, 36, 1569–1571 CAS.
- H. Kawai, Y. Hayakawa, M. Nakagawa, K. Furihata, K. Furihata, A. Shimazu, H. Seto and N. Otake, J. Antibiot., 1987, 40, 1266–1272 CAS.
- A. Shimosaka, H. Kawai, Y. Hayakawa, N. Komeshima, M. Nakagawa, H. Seto and N. Otake, J. Antibiot., 1987, 40, 1283–1291 CAS.
- H. Kawai, Y. Hayakawa, M. Nakagawa, K. Furihata, H. Seto and N. Otake, J. Antibiot., 1987, 40, 1273–1282 CAS.
- K. Ishigami, Y. Hayakawa and H. Seto, J. Antibiot., 1994, 47, 1219–1225 CAS.
- R. Kind, K. Hutter, A. Zeeck, K. Schmidt-Base and E. Egert, J. Antibiot., 1989, 42, 7–13 CAS.
- K. Hutter et al., J. Antibiot., 1986, 39, 1193–1204 CAS.
- T. J. Hosted, T. X. Wang, D. C. Alexander and A. C. Horan, J. Ind. Microbiol. Biotechnol., 2001, 27, 386–392 CrossRef CAS.
- Y. Hu, A. Al-Mestarihi, C. L. Grimes, D. Kahne and B. O. Bachmann, J. Am. Chem. Soc., 2008, 130, 15756–15757 CrossRef CAS.
- B. F. Hamilton, D. H. Gould and D. L. Gustine, in Mitochondrial Inhibitors and Neurodegenerative Disorders, ed. P. R. Sanberg, H. Nishino and C. V. Borlongan, Humana Press, Totowa, NJ, 1999, p. 21 Search PubMed.
- R. C. Anderson, W. Majak, M. A. Rassmussen, T. R. Callaway, R. C. Beier, D. J. Nisbet and M. J. Allison, J. Agric. Food Chem., 2005, 53, 2344–2350 CrossRef CAS.
- P. Chomcheon, S. Wiyakrutta, N. Sriubolmas, N. Ngamrojanavanich, D. Isarangkul and P. Kittakoop, J. Nat. Prod., 2005, 68, 1103–1105 CrossRef CAS.
- T. A. Alston, L. Mela and H. J. Bright, Proc. Natl. Acad. Sci. U. S. A., 1977, 74, 3767–3771 CrossRef CAS.
- C. J. Coles, D. E. Edmondson and T. P. Singer, J. Biol. Chem., 1979, 254, 5161–5167 CAS.
- M. C. Williams and R. C. Barneby, Brittonia, 1977, 29, 310–326 CrossRef CAS.
- M. C. Williams, F. R. Stermitz and R. D. Thomas, Phytochemistry, 1975, 14, 2306–2308 CrossRef CAS.
- X.-X. Zhang, Z.-X. Zhang, L. Chen and Y.-F. Su, Fitoterapia, 2006, 77, 15–18 CrossRef CAS.
- Y. Su, M. Lu, F. Yang, C. Li, L. Di, D. Wu, Z. Guo, J. Lu and D. Guo, Fitoterapia, 2008, 79, 451–455 CrossRef CAS.
- Y. Su, C. Li, Y. Gao, L. Di, X. Zhang and D. Guo, J. Nat. Prod., 2005, 68, 1785–1786 CrossRef CAS.
- M. Benn, Y. Bai and W. Majak, Phytochemistry, 1995, 40, 1629–1631 CrossRef CAS.
- K. Gorter, Bull. Jard. Bot., 1920, 2, 187–202 Search PubMed.
- C. L. Carter, J. Sci. Food Agric., 1951, 2, 54–55 CrossRef CAS.
- W. Majak and M. Benn, Phytochemistry, 1994, 35, 901–903 CrossRef CAS.
- B. G. Moyer, P. E. Pfeffer, K. M. Valentine and D. L. Gustine, Phytochemistry, 1979, 18, 111–113 CrossRef CAS.
- W. S. Garcez, F. R. Garcez and A. Barison, Biochem. Syst. Ecol., 2003, 31, 207–209 CrossRef CAS.
- W. S. Garcez, F. R. Garcez, N. K. Honda and A. J. R. da Silva, Phytochemistry, 1989, 28, 1251–1252 CrossRef CAS.
- W. Majak, M. Benn, D. McEwan and M. A. Pass, Phytochemistry, 1992, 31, 2393–2395 CrossRef CAS.
- W. Majak and R. J. Bose, Phytochemistry, 1976, 15, 415–417 CrossRef CAS.
- B. G. Moyer, P. E. Pfeffer, J. L. Moniot, M. Shamma and D. L. Gustine, Phytochemistry, 1977, 16, 375–377 CrossRef CAS.
- J. M. Pasteels, J. C. Braekman, D. Daloze and R. Ottinger, Tetrahedron, 1982, 38, 1891–1897 CrossRef CAS.
- M. H. Benn, W. Majak and R. Aplin, Biochem. Syst. Ecol., 1997, 25, 467–468 CrossRef CAS.
- M. A. Salem, J. Michael Williams, S. J. Wainwright and C. R. Hipkin, Phytochemistry, 1995, 40, 89–91 CrossRef CAS.
- F. R. Stermitz, F. A. Norris and M. C. Williams, J. Am. Chem. Soc., 1969, 91, 4599–4600 CrossRef CAS.
- M. H. Benn and W. Majak, Phytochemistry, 1989, 28, 2369–2371 CrossRef CAS.
- M. Long, M. Benn, W. Majak and R. McDiarmid, Phytochemistry, 1992, 31, 321–323 CrossRef CAS.
- W. Majak and M. H. Benn, Phytochemistry, 1988, 27, 1089–1091 CrossRef CAS.
- W. Majak, M. H. Benn and Y. Y. Huang, J. Nat. Prod., 1988, 51, 985–988 CrossRef CAS.
- R. L. Baxter, A. B. Hanley and H. W.-S. Chan, J. Chem. Soc., Chem. Commun., 1988, 757–758 RSC.
- E. Candlish, L. J. La Croix and A. M. Unrau, Biochemistry, 1969, 8, 182–186 CrossRef.
- D. Joulain, A. Casazza, R. Laurent, D. Portier, N. Guillamon, R. Pandya, M. Le and A. Viljoen, J. Agric. Food Chem., 2004, 52, 2322–2325 CrossRef.
- A. Krumbein and H. Auerswald, Nahrung, 1998, 42, 395–399 CrossRef CAS.
- B. A. Halkier and B. L. Møller, J. Biol. Chem., 1990, 265, 21114–21121 CAS.
- W. Hösel, J. Berlin, T. Hanzlik and E. Conn, Planta, 1985, 166, 176–181 CrossRef.
- F. Hanawa, S. Tahara and G. H. N. Towers, Phytochemistry, 2000, 53, 55–58 CrossRef CAS.
- H. Ina and H. Iida, Chem. Pharm. Bull., 1986, 34, 726–729 CAS.
- H. Shimomura, Y. Sashida, M. Oohara and H. Tenma, Phytochemistry, 1988, 27, 644–646 CrossRef CAS.
- M. Yoshikawa, S. Yoshizumi, T. Murakami, H. Matsuda, J. Yamahara and N. Murakami, Chem. Pharm. Bull., 1996, 44, 492–499 CAS.
- U. L. B. Jayasinghe, N. Hara and Y. Fujimoto, Nat. Prod. Res., 2007, 21, 260–264 CAS.
- Y. Su, Z. Zhang and C. Guo, Fitoterapia, 2004, 75, 420–422 CrossRef CAS.
- B.-W. Yu, J.-Y. Chen, T.-X. Zhou, K.-F. Cheng and G.-W. Qin, Nat. Prod. Lett., 2002, 16, 155–159 CAS.
- V. Pabuccuoglu, G. Arar, T. Gözler, A. J. Freyer and M. Shamma, J. Nat. Prod., 1989, 52, 716–719 CrossRef.
- R. F. Bond, J. C. A. Boeyens, C. W. Holzapfel and P. S. Steyn, J. Chem. Soc., Perkin Trans. 1, 1979, 1751–1761 RSC.
- C. W. Holzapfel, Tetrahedron, 1968, 24, 2101–2119 CrossRef.
- R. R. Manam, S. Teisan, D. J. White, B. Nicholson, J. Grodberg, S. T. C. Neuteboom, K. S. Lam, D. A. Mosca, G. K. Lloyd and B. C. M. Potts, J. Nat. Prod., 2005, 68, 240–243 CrossRef CAS.
- J. Meinwald, J. Smolanoff, A. T. McPhail, R. W. Miller, T. Eisner and K. Hicks, Tetrahedron Lett., 1975, 16, 2367–2370 CrossRef.
- R. W. Miller and A. T. McPhail, J. Chem. Res., 1978, 1978, 76 Search PubMed.
- J. Smolanoff, A. F. Kluge, J. Meinwald, A. McPhail, R. W. Miller, K. Hicks and T. Eisner, Science, 1975, 188, 734–736 CAS.
- S. G. Spanton and G. D. Prestwich, Tetrahedron, 1982, 38, 1921–1930 CrossRef CAS.
- R. Piskorski, R. Hanus, S. Vašíčková, J. Cvačka, J. Šobotník, A. Svatoš and I. Valterová, J. Chem. Ecol., 2007, 33, 1787–1794 CrossRef CAS.
- P. R. S. Baker, Y. Lin, F. J. Schopfer, S. R. Woodcock, A. L. Groeger, C. Batthyany, S. Sweeney, M. H. Long, K. E. Iles, L. M. S. Baker, B. P. Branchaud, Y. E. Chen and B. A. Freeman, J. Biol. Chem., 2005, 280, 42464–42475 CrossRef CAS.
- P. R. S. Baker, F. J. Schopfer, S. Sweeney and B. A. Freeman, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 11577–11582 CrossRef CAS.
- J. A. Palermo, M. F. Rodriguez Brasco, C. Spagnuolo and A. M. Seldes, J. Org. Chem., 2000, 65, 4482–4486 CrossRef CAS.
- D. R. Hall, P. S. Beevor, D. G. Campion, D. J. Chamberlain, A. Cork, R. D. White, A. Almestar and T. J. Henneberry, Tetrahedron Lett., 1992, 33, 4811–4814 CrossRef CAS.
- B. J. Novak, D. R. Blake, S. Meinardi, F. S. Rowland, A. Pontello, D. M. Cooper and P. R. Galassetti, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 15613–15618 CrossRef CAS.
- Y. Miyazaki, Y. Kono, A. Shimazu, S. Takeuchi and H. Yonehara, J. Antibiot., 1968, 21, 279–282 CAS.
- W. S. Chilton and C. P. Hsu, Phytochemistry, 1975, 14, 2291–2292 CrossRef CAS.
- S. Hatanaka, Trans. Mycol. Soc. Jpn., 1981, 22, 213–217 Search PubMed.
- N. A. Bruender, J. B. Thoden and H. M. Holden, Biochemistry, 2010, 49, 3517–3524 CrossRef CAS.
- J. L. Vey, A. Al-Mestarihi, Y. Hu, M. A. Funk, B. O. Bachmann and T. M. Iverson, Biochemistry, 2010, 49, 9306–9317 CrossRef CAS.
- S. C. Timmons and J. S. Thorson, Curr. Opin. Chem. Biol., 2008, 12, 297–305 CrossRef CAS.
- S. Schmidt, J. F. Blom, J. Pernthaler, G. Berg, A. Baldwin, E. Mahenthiralingam and L. Eberl, Environ. Microbiol., 2009, 11, 1422–1437 CrossRef.
| This journal is © The Royal Society of Chemistry 2011 |