Potential atypical antipsychotics: synthesis, binding affinity and SAR of new heterocyclic bioisosteric butyrophenone analogues as multitarget ligands†
María
Barceló
a,
Enrique
Raviña
a,
María J.
Varela
b,
José
Brea
b,
María I.
Loza
b and
Christian F.
Masaguer
*a
aDepartamento de Química Orgánica, Laboratorio de Química Farmacéutica, Facultad de Farmacia, Universidad de Santiago de Compostela, E-15782, Santiago de Compostela, Spain. E-mail: christianf.masaguer@usc.es; Fax: +34-881-594628; Tel: +34-881-815099
bDepartamento de Farmacología, Facultad de Farmacia, Universidad de Santiago de Compostela, E-15782, Santiago de Compostela, Spain
First published on 11th October 2011
Abstract
A series of 15 heterocyclic butyrophenone analogues was synthesized as potential multi-target ligands. The compounds were assayed for their in vitro human D2 and 5-HT2A receptor affinity, and those compounds exhibiting the highest affinities were evaluated for binding affinity on D1, D3, 5-HT1A, 5-HT2C and 5-HT6 receptors.
Introduction
Schizophrenia is a complex neuropsychiatric disorder characterized by the development of three distinct kinds of symptoms: positive (hallucinations, hyperactivity, delusions, disorganized speech), negative (anhedonia, avolition, social isolation), and cognitive (attentional impairment, memory deficits). For the treatment of this disorder, the first generation of antipsychotic drugs was discovered about fifty years ago (e.g.chlorpromazine, haloperidol); described as selective D2 dopamine antagonists, today they are known as “typical antipsychotics”.1 These drugs produce however a host of adverse side effects including induction of acute extrapyramidal symptoms (EPS), without having any effect on the negative or cognitive symptoms of schizophrenia.2 Later, the introduction of clozapine for treatment-resistant schizophrenia gave rise to a new group of “atypical” or “nonclassical” antipsychotics that have no EPS at the doses frequently used in therapy, and are effective also against the negative symptoms.3–5 A new generation of atypical antipsychotics (also known as “dopamine stabilizers”) such as aripiprazole, with a concrete efficacy spectrum in different receptors (inverse agonist at 5-HT2B receptors, partial agonist at D2, D3, 5-HT2A and 5-HT2C receptors),6 offered further advantage due to an improved efficacy in treating negative symptoms of schizophrenia and a decreased incidence and severity of central and peripheral side effects.7 Now, while the treatment and maintenance of the positive symptoms of schizophrenia are largely addressed with current medications, the major challenge is to identify compounds showing clinically significant improvements in the treatment of negative symptoms and cognitive dysfunction. In addition, a major issue with many of the currently prescribed atypical antipsychotic drugs remains the side-effect liabilities of weight gain, metabolic abnormalities, diabetes liabilities and potential cardiovascular safety concerns and so significant improvements can still be made. Consequently, much of the current focus in the design of new antipsychotic drugs has been centered on trying to improve upon these liabilities.At the molecular level, while traditional antipsychotics bind principally and most avidly to D2 receptors, atypical antipsychotics exhibit high affinities for other receptors equal to or exceeding that for the D2receptor; clozapine and the closely related compound olanzapine are good examples of drugs with a complex multi-receptor profile; they have affinities toward serotonin, dopamine, α-adrenergic, muscarinic, and histamine receptors, among others.8 Moreover, the second-generation atypical antipsychotics are partial agonists of D2 and 5-HT1A receptors, and antagonists of 5-HT2A receptors and also a number of other targets. In general, experimental evidence suggests that a complex binding profile is linked to the clinical efficacy of antipsychotic drugs, and indeed, the importance of multivalent ligands targeting G-protein-coupled receptors design to deal with schizophrenia has been pointed by many authors.9,10 In fact, some of the latest efforts in the development of novel antipsychotic drugs are aimed at obtaining compounds with binding affinities for a certain number of receptors.11–14 To validate this multireceptor affinity profile approach to antipsychotics and to achieve an optimum interaction with dopamine and serotonin receptors, we exploited our knowledge acquired in the development of aminobutyrophenone potential antipsychotics. Herein we report on the design, synthesis, and pharmacological evaluation of new aminobutyrophenone heterocyclic derivatives as multi-target compounds potentially useful as antipsychotics.
Over the last years, we have been working on the modulation of the butyrophenone system, with the aim of combining the antagonism at the 5-HT2 family and the D2 receptors in a single molecule.15–18 We have described a series of aminobutyrophenones type I and II (Fig. 1, substructure marked bold) which showed high affinity for the 5-HT2A receptor subtype, with pKi values between 7.24 and 8.37.19,20 We explored now the possibility of synthesizing analogues of the above mentioned aminobutyrophenones, in which the furan or pyrrole ring of these compounds was replaced by five-membered heterocycles containing two heteroatoms: pyrazole, isoxazole, oxazole, and thiazole, to form a tetrahydro-indazolone, -benzisoxazolone, -benzoxazolone, or -benzothiazolone system, respectively, considering that the substitution of –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) by –N
by –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) in aromatic rings has been one of the most-successful applications of classical isosterism.21–23 These modifications should deliver insight into the dependency of the receptor binding efficacies on the number of heteroatoms in the ring system and their position there. The isosteric replacement of the benzene ring at the butyrophenone motif resulted in 15 analogues with different substitutions. Some preliminary results of this work have been published in a previous communication.24
in aromatic rings has been one of the most-successful applications of classical isosterism.21–23 These modifications should deliver insight into the dependency of the receptor binding efficacies on the number of heteroatoms in the ring system and their position there. The isosteric replacement of the benzene ring at the butyrophenone motif resulted in 15 analogues with different substitutions. Some preliminary results of this work have been published in a previous communication.24
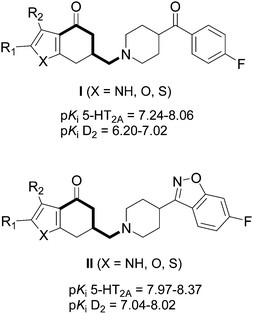 | ||
| Fig. 1 Structures of some heterocyclic aminobutyrophenone analogues. | ||
Chemistry
The starting product for the synthesis of the target compounds 11–17, 22, and 27 (Schemes 1–3) was 5-(methoxymethyl)-cyclohexane-1,3-dione 1, which was prepared from the commercial 3,5-dimethoxy- (or 3,4,5-trimethoxy-) benzoic acid in a four step procedure, as previously described by our research laboratory.25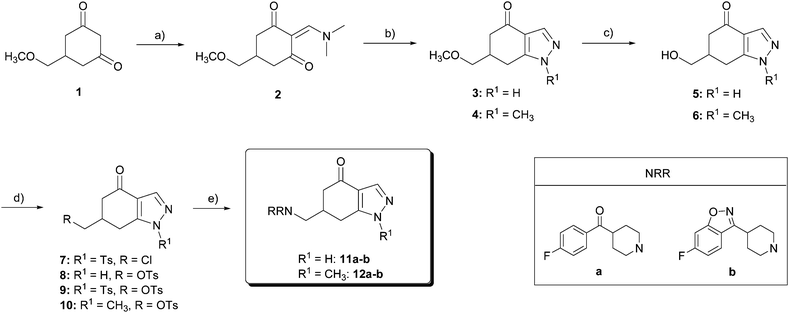 | ||
| Scheme 1 Reagents and conditions: (a) DMFDMA, THF, 80 °C, 3 h, 95%; (b) Ac2O, Et3N, DMAP, CHCl3, r.t., 24 h, 69%; (c) NH2NH2·2HCl, NaOH, MeOH, 80 °C, 4 h, 70%; (d) NH2NHCH3, MeOH, 80 °C, 2.5 h, 70%; (e) NH2OH·HCl, KOH, MeOH, C6H6, r.t., 48 h, 71%; (f) BBr3, CH2Cl2, −40 °C → r.t., 24 h, 40–81%; (g) BBr3, 15-crown-5, CH2Cl2, −30 °C, 4 h, 56%; (h) TsCl, Py, r.t., 24 h, 50–71%; and (i) HNRR, solvent, reflux. | ||
For the synthesis of indazolones 11 and 12 (Scheme 1), diketone 1 was condensed with N,N-dimethylformamide dimethylacetal (DMFDMA) to give amino ketone 2 in 95% yield. By a Michael addition–elimination/cyclodehydration process, using hydrazine dihydrochloride and NaOH at reflux, 2 was transformed into pyrazole 3 in 70% yield, and methylhydrazine at reflux afforded 4 as a single regioisomer, also in 70% yield. Methyl ethers of 3 and 4 were cleaved with BBr3 in CH2Cl2 at −40 °C → r.t., affording the corresponding alcohols 5 and 6 in 81% and 40% yield, respectively. Forcing these conditions (2 mol equiv. BBr3, higher temperatures, or longer reaction times) or using other reagents, such as trimethylsilane iodide, gave complex mixtures of products.
Tosylation of 5 was complicated due to the reaction between the unprotected nitrogen of the pyrazole ring and the tosyl chloride: first attempts for tosylation of the hydroxyl group led to a mixture of chloride 7, monotosylate 8 and ditosylate 9. Then, we decided to increase the number of equivalents of tosyl chloride to force the formation of the ditosyl derivative 9, to further deprotect the pyrazole ring. Under these conditions, ditosylate 9 was obtained in 50% yield as the major product. Tosylation of alcohol 6 under standard conditions furnished the corresponding sulfonate 10 in 71% yield. Tosylates 9 and 10 were converted into the required amines 11–12 by nucleophilic displacement of the tosyl group by the corresponding substituted piperidines a or b (Scheme 1). Nucleophilic substitution reaction on ditosylate 9 proceeded with simultaneous attack at the electron-deficient sulfur atom of the N-tosylpyrazole, which resulted in deprotection of the pyrazole ring, affording the desired amines 11a and 11b in a single step.24
On the other hand, 1 was converted into a 2-acetyl derivative 13 (Scheme 2) by treatment with acetic anhydride and triethylamine in the presence of 4-(dimethylamino)pyridine (DMAP) in 69% yield. Condensation of 13 with hydroxylamine hydrochloride in the presence of KOH yielded 14 in 71%. Methylether cleavage in benzisoxazolone 14 with BBr3 led to alcohol 15 in a modest 30% yield. The addition of 15-crown-5 to BBr3 allowed us to improve the yield up to 56%. Tosylation of alcohol 15 gave the corresponding sulfonate 16 in 61% yield, and nucleophilic displacement of the tosyl group by the corresponding substituted piperidines a, b or c furnished amines 17a–c.
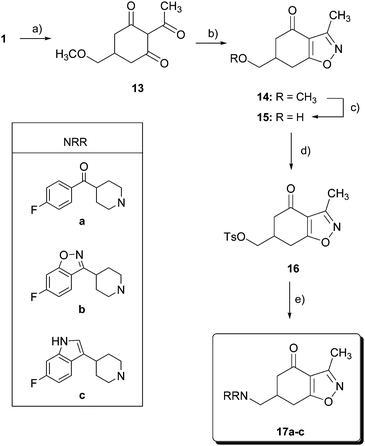 | ||
| Scheme 2 Reagents and conditions: (a) Ac2O, Et3N, DMAP, CHCl3, r.t., 24 h, 69%; (b) NH2OH·HCl, KOH, MeOH, C6H6, r.t., 48 h, 71%; (c) BBr3, 15-crown-5, CH2Cl2, −30 °C, 4 h, 56%; (d) p-TsCl, pyridine, 0 °C, 12 h, 61%; and (e) HNRR, CH3CN, reflux. | ||
For the synthesis of oxazolones 22 (Scheme 3), the construction of the oxazole ring was tackled by rhodium-catalyzed cyclocondensation of a diazocarbonyl derivative with a nitrile, following the method reported by Lee and Suk.26 Thus, reaction of compound 1 with tosylazide in the presence of Et3N afforded the diazodicarbonyl derivative 18 (IR peak at 2142 cm−1) in 80% yield. Dipolar cycloaddition of 18 with acetonitrile (as the solvent and reagent) in the presence of 2 mol% of Rh2(OAc)4 at reflux gave 6,7-dihydro-2-methyl-6-(methoxymethyl)-benzoxazol-4(5H)-one (19) in 60% yield. As described above, methylether cleavage of tetrahydrobenzoxazolone 19 with 1 M solution of BBr3 led to alcohol 20 in 65% yield. Tosylation of 20 (66% yield) and subsequent reaction with amines a–d furnished final tetrahydrobenzoxazolones 22a–d in 20–50% yield.
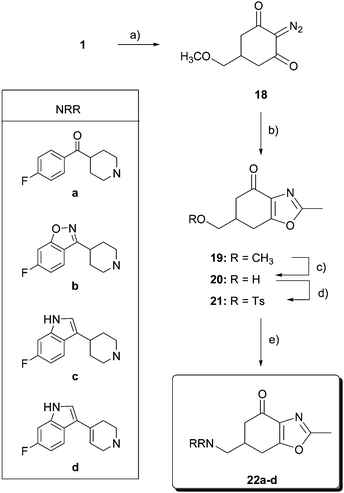 | ||
| Scheme 3 Reagents and conditions: (a) p-tosyl azide, Et3N, CH2Cl2, r.t., 15 h, 80%; (b) Rh2(OAc)4, CH3CN, 60 °C, 7 h, 60%; (c) BBr3, CH2Cl2, −40 °C, 12 h, 0 °C, 12 h, 65%; (d) p-TsCl, pyridine, 0 °C, 12 h, 66%; and (e) HNRR, CH3CN, reflux. | ||
Benzothiazolones 27 (Scheme 4) were prepared using thiourea as a source of heteroatoms for the construction of the thiazole ring. Accordingly, one pot reaction of diketone 1 with thiourea, NBS and benzoyl peroxide (catalytic) in benzene gave the benzothiazolone 23 in 70% yield. The amino group was alkylated with 1,4-dibromobutane in acetone in the presence of NaOH to afford 5,6-dihydro-5-(methoxymethyl)-2-(pyrrolidin-1-yl)benzo-thiazol-7(4H)-one (24) in 70% yield. From methyl ether 24, the same three-step procedure described above yielded the final aminomethylbenzothiazolones 27a–d.
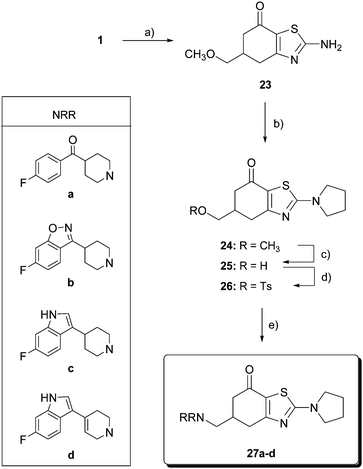 | ||
| Scheme 4 Reagents and conditions: (a) thiourea, NBS, benzoyl peroxide, benzene, reflux, 48 h, 70%; (b) 1,4-dibromobutane, NaOH, reflux, 7 h, 70%; (c) BBr3, CH2Cl2, −40 °C, 12 h, then 0 °C, 12 h, 91%; (d) p-TsCl, pyridine, 0 °C, 12 h, 57%; and (e) HNRR, CH3CN, reflux. | ||
Receptor binding studies
One way to distinguish the atypical antipsychotics from the conventional agents is that they block 5-HT2A receptors as well as D2 receptors and have fewer motor side effects such as EPS than the conventional antipsychotics have at standard doses. On this basis, the affinity of the compounds 11, 12, 17, 22 and 27 for cloned human D2 and 5-HT2A receptors was first evaluated in in vitro binding assays using the radioligands [3H]spiperone and [3H]ketanserin, respectively, according to our previously described procedures.27 Affinity values (expressed as pKi) were calculated according to the Cheng–Prusoff equation,28 and the in vitroreceptor binding data are summarized in Table 1.| Compound | Structure | D2 | 5-HT2A | pKi 5-HT2A/pKi D2 |
|---|---|---|---|---|
| a Values are means of two separate experiments. b Value taken from ref. 43. | ||||
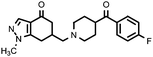
| 11a (QF4104B) |

|
6.40 ± 0.15 | 7.37 ± 0.22 | 1.15 |
| 11b (QF4108B) |

|
6.95 ± 0.06 | 8.67 ± 0.19 | 1.25 |
| 12a (QF4124B) |
|
1.4% | 7.52 ± 0.16 | — |
| 12b (QF4128B) |

|
10.9% | 7.76 ± 0.44 | — |
| 17a (QF4214B) |

|
<5 | 7.39 ± 0.12 | — |
| 17b (QF4218B) |

|
6.92 ± 0.3 | 7.76 ± 0.32 | 1.12 |
| 17c (QF4216B) |

|
7.20 ± 0.10 | 8.00 ± 0.09 | 1.11 |
| 22a (QF4414B) |

|
35% | 6.90 ± 0.10 | — |
| 22b (QF4418B) |

|
7.49 ± 0.06 | 7.70 ± 0.05 | 1.03 |
| 22c (QF4416B) |

|
6.70 ± 0.20 | 8.30 ± 0.05 | 1.24 |
| 22d (QF4417B) |

|
6.76 ± 0.12 | 7.10 ± 0.10 | 1.05 |
| 27a (QF4514B) |

|
30% | 6.55 ± 0.20 | — |
| 27b (QF4518B) |

|
6.70 ± 0.08 | 7.70 ± 0.20 | 1.15 |
| 27c (QF4516B) |
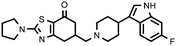
|
6.80 ± 0.10 | 8.10 ± 0.09 | 1.19 |
| 27d (QF4517B) |

|
7.26 ± 0.13 | 7.40 ± 0.09 | 1.02 |
| Haloperidol | 9.22 ± 0.12 | 6.78 ± 0.25 | 0.73 | |
| Clozapine | 6.65 ± 0.17 | 8.04 ± 0.31 | 1.21 | |
| Risperidone | 8.21b | 9.30 ± 0.25 | 1.13 |
In general, the synthesized compounds showed greater affinity for the serotonin 5-HT2Areceptor (pKi range of values between 6.55, for 27a, and 8.67, for 11b) than dopamine D2 receptors. Furthermore, in those series in which it has been incorporated, the fragment fluoroindolylpiperidine led to compounds with higher affinity for the two receptors tested. We can establish a decreasing order of affinities for the four amines used in the receptors tested that, with a few exceptions, is fulfilled in the synthesized compounds: c > b > d > a.
Compounds bearing the 4-(4-fluorobenzoyl)piperidine fragment (Table 1) have low or no affinity for dopamine D2 receptors, except the pyrazole derivative 11a, with a pKi D2 = 6.40, which is the only one of the five compounds tested containing a hydrogen donor group in its heterocyclic system. This fact points to the existence of an interaction with the D2receptor binding site that would not occur in the rest of the compounds. Judging from the dopaminergic hypothesis of schizophrenia, those compounds without affinity for the D2receptor could not be considered as potential antipsychotics. As regards the 5-HT2A receptors, some derivatives have moderate affinity for this receptor, with pKi values ranging from 6.55 to 7.52, the N-methylpyrazole derivative 12a being the compound with higher affinity in this series.
Compounds bearing a 4-(benzoisoxazolyl)piperidine system generally have a higher affinity for the two receptors studied than their counterparts that contain a benzoylpiperidine (Table 1). Therefore, the interaction between the benzoisoxazole system and the receptor binding site must be stronger than that of the benzoyl system, such that those compounds with no affinity now have affinity for D2 (17avs.17b; 22avs.22b; 27avs.27b) receptors. Among the five benzoisoxazolylpiperidine derivatives synthesized, compound 11b, from the point of view of the potential antipsychotic activity would show, based on the Meltzer index,29 an atypical profile (pKi5-HT2A/pKi D2 = 1.25). Furthermore, its profile is similar to that shown for clozapine.
Compounds bearing a 4-(6-fluoroindol-3-yl)piperidine (17c, 22c and 27c) were those that showed higher affinity at the 5-HT2A receptors, while unsaturated analogues 22d and 27d, containing a tetrahydropyridine moiety, have lower affinity at 5-HT2A receptors, but higher affinity at D2 receptors, probably due to the different spatial arrangement that takes the indole ring when it is attached to a piperidine or to a tetrahydropyridine ring, and thus different interactions in the binding site of the receptors studied.
The indolylpiperidine derivatives 22c and 27c (together with the above mentioned benzoisoxazolylpiperidine 11b) also showed a Meltzer index predictive of an atypical profile, making them candidate compounds for further pharmacological study to determine the affinity profile on other receptors involved in the antipsychotic effect.
Apart from D2 and 5-HT2A receptors, other GPCRs have been proposed to be involved in the antipsychotic effect. The dopamine D1 receptor plays an important role in mediating dopaminergic transmission in the prefrontal cortex, which is decreased in schizophrenia patients. Controversy about this topic still exists despite ample evidence suggesting that the D1receptor gene is associated with performance on neuropsychological tests probing the function of the prefrontal cortex in schizophrenia, as well as positive and negative symptoms and therapeutic response to antipsychotics. As an important dopaminergic gene, the D1receptor may contribute to schizophrenia by interacting with other genes.30
Dopamine, through D3 receptors, modulates the cholinergic system at the prefrontal cortex level. Although the D3receptor involvement in antipsychotic therapy is not already clear, D3antagonists (devoid of muscarinic effects) may enhance acetylcholine release in the frontal cortex,31 thus improving cognitive deficits poorly treated by currently available agents.32
5-HT1A affinity contributes to the clinical efficacy of most of the atypical antipsychotics (aripiprazole, clozapine, olanzapine) and their low liability for EPS.33,34 Moreover, there are several clinical studies which indicate that 5-HT1A agonism may be beneficial to improve cognition in some patients with schizophrenia.35 Indeed, it has been proposed that 5-HT1A receptor stimulation, as well 5-HT6 and 5-HT7 antagonism, may contribute to the beneficial effects in cognition of atypical antipsychotic drugs.36
Evidence suggests that 5-HT2C receptors play a role in schizophrenia.37,385-HT2C receptor antagonists have been shown to reduce neuroleptic-induced catalepsy and thus it has been suggested that antagonism of 5-HT2C receptors may be responsible for the reduced risk of extrapyramidal side effects associated with the atypical antipsychotic medications,39 and also affects cognition and psychosis by modulating the cortical and limbic dopaminergic activity.36
On the other hand, neurobiology and in vivo pharmacology studies in rodents have shown that 5-HT6 antagonists improve memory and cognition through involvement in multiple neurotransmission systems including both cholinergic and glutamatergic systems and, therefore, can potentially provide an effective treatment for cognitive dysfunction in schizophrenia.36,40,41
Based on this evidence as well as their internal experience, Payne proposed five key receptors for antagonism for the treatment of schizophrenia, proposing that a compound should have pKi ≥ 8 at D3, 5-HT2A, 5-HT2C and 5-HT6 receptors, and pKi ≥ 7 at D2 receptors for showing atypical antipsychotic behaviour.11,42
Taking all these into account, the binding profile of the three selected compounds was tested over these receptors (Table 2).
| Compound | D1 | D3 | 5-HT1A | 5-HT2C | 5-HT6 |
|---|---|---|---|---|---|
| 11b (QF4108B) | 7.41 | 6.73 | 5.82 | 6.91 | 6.20 |
| 22c (QF4416B) | 7.47 | 5.87 | 6.31 | 7.00 | 6.67 |
| 27c (QF4516B) | 7.44 | 5.96 | 6.99 | 6.90 | 6.45 |
The three selected compounds have an equivalent selectivity profile, with similar affinities on the seven receptors tested: 5-HT2A > D1 > D2 ≈ D3 ≈ 5-HT2C ≈ 5-HT6 ≈ 5-HT1A. The binding profile was close to that proposed for the five key receptors, with the only exception of D3 receptors where compounds 22c and 27c showed pKi values of 5.87 and 5.96, respectively, vs. a proposed pKi ≥ 8, while compound 11b showed a pKi value of 6.73 at D3 receptors, closer to the proposed one for an atypical antipsychotic profile. In addition to their interest as leads for antipsychotics, the subtle differences observed in their profile make these compounds very interesting as pharmacological tools because they could allow to ascertain the involvement of 5-HT6 and, overall, D3 receptors in the antipsychotic pharmacology.
Conclusions
In this work we describe the synthesis, binding affinity and SAR of new heterocyclic bioisosteric butyrophenone analogues as multi-target ligands. For the 15 compounds synthesized, binding affinity assays on D2 receptors showed that compounds with higher affinity were those bearing an indolylpiperidine system followed by those containing a benzisoxazolylpiperidine fragment. With respect to the heterocycle fused to the cyclohexanone, the isoxazole ring afforded the highest affinity compounds. For the 5-HT2A receptor, the trend observed in the piperidine moiety seems the same to that for the dopamine D2 receptor: indolyl > benzisoxazolyl > benzoyl. In relation to the fused heterocyclic ring, available data showed a higher affinity for the pyrazole derivatives. Moreover, in the thiazole series, the presence of a bulky amine such as pyrrolidine did not seem to have a great influence on the affinity for this receptor. In particular, among the compounds prepared, 11b, 22c and 27c exhibited the highest affinity for binding to the D2 and 5-HT2A receptors, and consequently the affinities on D1, D3, 5-HT1A, 5-HT2C and 5-HT6 receptors were determined for these three compounds. The affinities of the selected compounds on the receptors assayed could allow to ascertain the involvement of 5-HT6 and D3 receptors in the antipsychotic effect.Acknowledgements
This work was supported by Spanish Ministerio de Educación y Ciencia (Grants SAF2005-08025-C03 and SAF2009-13609-C04) and Xunta de Galicia (Grant INCITE09 203 184PR). A PhD fellowship (FPU) has been granted to M. Barceló by the Ministerio de Educación y Ciencia.Notes and references
- B. L. Roth, D. J. Sheffler and W. K. Kroeze, Nat. Rev. Drug Discovery, 2004, 3, 353–359 CrossRef CAS.
- A. Farah, Prim. Care Companion. J. Clin. Psychiatry, 2005, 7, 268–274 CrossRef.
- A. Fitton and R. C. Heel, Drugs, 1990, 40, 722–747 CrossRef CAS.
- J. T. Schwarz and A. W. Brotman, Drugs, 1992, 44, 981–992 CrossRef.
- R. Rosenheck, J. Cramer, W. Xu, J. Thomas, W. Henderson, L. Frisman, C. Fye and D. N. Charney, N. Engl. J. Med., 1997, 337, 809–815 CrossRef CAS.
- D. A. Shapiro, S. Renock, E. Arrington, L. A. Chiodo, L. X. Liu, D. R. Sibley, B. L. Roth and R. Mailman, Neuropsychopharmacology, 2003, 28, 1400–1411 CrossRef CAS.
- S. M. Stahl, J. Clin. Psychiatry., 1999, 60(Suppl. 10), 31–41 Search PubMed.
- S. M. Stahl and M. M. Grady, Curr. Med. Chem., 2004, 11, 313–327 CrossRef CAS.
- R. Morphy and Z. Rankovic, Curr. Pharm. Des., 2009, 15, 587–600 CrossRef CAS.
- E. H. Wong, F. I. Tarazi and M. Shahid, Pharmacol. Ther., 2010, 126, 173–185 CrossRef CAS.
- V. Garzya, I. T. Forbes, A. D. Gribble, M. S. Hadley, A. P. Lightfoot, A. H. Payne, A. B. Smith, S. E. Douglas, D. G. Cooper, I. G. Stansfield, M. Meeson, E. E. Dodds, D. N. C. Jones, M. Wood, C. Reavill, C. A. Scorer, A. Worby, G. Riley, P. Eddershaw, C. Ioannou, D. Donati, J. J. Hagan and E. A. Ratti, Bioorg. Med. Chem. Lett., 2007, 17, 400–405 CrossRef CAS.
- G. Neves, R. Menegatti, C. B. Antonio, L. R. Grazziottin, R. O. Vieira, S. M. K. Rates, F. Noël, E. J. Barreiro and C. A. M. Fraga, Bioorg. Med. Chem., 2010, 18, 1925–1935 CrossRef CAS.
- S. Butini, S. Gemma, G. Campiani, S. Franceschini, F. Trotta, M. Borriello, N. Ceres, S. Ros, S. Sanna Coccone, M. Bernetti, M. De Angelis, M. Brindisi, V. Nacci, I. Fiorini, E. Novellino, A. Cagnotto, T. Mennini, K. Sandager-Nielsen, J. T. Andreasen, J. Scheel-Kruger, J. D. Mikkelsen and C. Fattorusso, J. Med. Chem., 2009, 52, 151–169 CrossRef CAS.
- S. Butini, G. Campiani, S. Franceschini, F. Trotta, V. Kumar, E. Guarino, G. Borrelli, I. Fiorini, E. Novellino, C. Fattorusso, M. Persico, N. Orteca, K. Sandager-Nielsen, T. A. Jacobsen, K. Madsen, J. Scheel-Kruger and S. Gemma, J. Med. Chem., 2010, 53, 4803–4807 CrossRef CAS.
- E. Raviña and C. F. Masaguer, Curr. Med. Chem.: Cent. Nerv. Syst. Agents, 2001, 1, 43–62 CrossRef.
- Y. Caro, M. Torrado, C. F. Masaguer, E. Raviña, F. Padín, J. Brea and M. I. Loza, Bioorg. Med. Chem. Lett., 2004, 14, 585–589 CrossRef CAS.
- C. Dezi, J. Brea, M. Alvarado, E. Ravina, C. F. Masaguer, M. I. Loza, F. Sanz and M. Pastor, J. Med. Chem., 2007, 50, 3242–3255 CrossRef CAS.
- R. Aranda, K. Villalba, E. Raviña, C. F. Masaguer, J. Brea, F. Areias, E. Domínguez, J. Selent, L. López, F. Sanz, M. Pastor and M. I. Loza, J. Med. Chem., 2008, 51, 6085–6094 CrossRef CAS.
- C. F. Masaguer, E. Raviña, I. Loza and J. A. Fontenla, Bioorg. Med. Chem. Lett., 1997, 7, 913–918 CrossRef CAS.
- J. Brea, J. Rodrigo, A. Carrieri, F. Sanz, M. I. Cadavid, M. J. Enguix, M. Villazón, G. Mengod, Y. Caro, C. F. Masaguer, E. Raviña, N. B. Centeno, A. Carotti and M. I. Loza, J. Med. Chem., 2002, 45, 54–71 CrossRef CAS.
- P. Ciapetti and B. Giethlen, in The Practice of Medicinal Chemistry, ed. C. G. Wermuth, Prestwick Chemical Inc., Illkirch, 3rd edn, 2008, pp. 294–323 Search PubMed.
- G. A. Patani and E. J. LaVoie, Chem. Rev., 1996, 96, 3147–3176 CrossRef CAS.
- L. B. Kier and L. H. Hall, Chem. Biodiversity, 2004, 1, 138–151 CAS.
- M. Barcelo, E. Raviña, C. F. Masaguer, E. Dominguez, F. M. Areias, J. Brea and M. I. Loza, Bioorg. Med. Chem. Lett., 2004, 17, 4873–4877 CrossRef.
- B. Pita, C. F. Masaguer and E. Raviña, Tetrahedron Lett., 2000, 41, 9829–9833 CrossRef CAS.
- Y. R. Lee and J. Y. Suk, Heterocycles, 1998, 48, 875–883 CrossRef CAS.
- J. Brea, M. Castro, M. I. Loza, C. F. Masaguer, E. Raviña, C. Dezi, M. Pastor, F. Sanz, A. Cabrero-Castel, B. Galán-Rodríguez, E. Fernández-Espejo, R. Maldonado and P. Robledo, Neuropharmacology, 2006, 51, 251–262 CrossRef CAS.
- Y.-C. Cheng and W. H. Prusoff, Biochem. Pharmacol., 1973, 22, 3099–3108 CrossRef CAS.
- H. Y. Meltzer, S. Matsubara and J. C. Lee, J. Pharmacol. Exp. Ther., 1989, 251, 238–246 CAS.
- C. Zhang, Y. Fang, B. Xie, W. Cheng, Y. Du, D. Wang and S. Yu, Psychiatry Research, 2010, 177, 350–353 CrossRef CAS.
- M. J. Millan, F. Loiseau, A. Dekeyne, A. Gobert, G. Flik, T. I. Cremers, J. M. Rivet, D. Sicard, R. Billiras and M. Brocco, J. Pharmacol. Exp. Ther., 2008, 324, 1212–1226 CrossRef CAS.
- L. P. Lacroix, M. E. Hows, A. J. Shah, J. J. Hagan and C. A. Heidbreder, Neuropsychopharmacology, 2003, 28, 839–849 CAS.
- E. Richelson and T. Souder, Life Sci., 2000, 68, 29–39 CrossRef CAS.
- M. J. Millan and J. Pharmacol, Exp. Ther., 2000, 295, 853–861 CAS.
- H. Y. Meltzer and T. Sumiyoshi, Behav. Brain Res., 2008, 195, 98–102 CrossRef CAS.
- H. Y. Meltzer and B. W. Massey, Curr. Opin. Pharmacol., 2001, 11, 59–67 CrossRef.
- H. Y. Meltzer, Neuropsychopharmacology, 1999, 21, 106S–115S CAS.
- V. J. Aloyo, K. A. Berg, U. Spampinato, W. P. Clarke and J. A. Harvey, Pharmacol. Ther., 2009, 121, 160–173 CrossRef CAS.
- C. Reavill, A. Kettle, V. Holland, G. Riley and T. P. Blackburn, Br. J. Pharmacol., 1999, 126, 572–574 CrossRef CAS.
- K. G. Liu and A. J. Robichaud, Drug Dev. Res., 2009, 70, 145–168 CrossRef CAS.
- G. Rosse and H. Schaffhauser, Curr. Top. Med. Chem., 2010, 10, 207–221 CrossRef CAS.
- C. Castagnoli, G. Gentile and A. H. Payne, Preparation of 7-[4-(3-fluoro-4-methylbenzyl)benzenesulfonyl]-8-methoxy-3-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine and related pharmaceutical salt compounds as antipsychotic agents, WO 2005051399.
- A. Schotte, P. F. Janssen, W. Gommeren, W. H. Luyten, P. Van Gompel, A. S. Lesage, K. DeLoore and J. E. Leysen, Psychopharmacology, 1996, 124, 57–73 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and spectroscopic data. See DOI: 10.1039/c1md00202c |
| This journal is © The Royal Society of Chemistry 2011 |
