SBA-15-Pr–SO3H as nanoreactor catalyzed oxidation of sulfides into sulfoxides†
Kiumars
Bahrami
*ab,
Mohammad M.
Khodaei
*ab and
Peyman
Fattahpour
a
aDepartment of Chemistry, Razi University, Kermanshah 67149-67346, Iran
bNanoscience and Nanotechnology Research Center (NNRC), Razi University, Kermanshah, 67149-67346, Iran. E-mail: kbahrami2@hotmail.com; mmkhoda@razi.ac.ir; Fax: +98(831)4274559; Tel: +98(831)4274559
First published on 29th March 2011
Abstract
The selective oxidation of sulfides to sulfoxides was achieved in excellent yields at 40 °C with SBA-15-Pr–SO3H as a recyclable nanoreactor active catalyst and H2O2 as the terminal oxidant. The H2O2–SBA-15-Pr–SO3H system can chemoselectively oxidize alkyl and aryl sulfides in the presence of oxidatively sensitive functional groups such as C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, –CN, and –OH.
C, –CN, and –OH.
Mesoporous materials have attracted considerable attention since they were first reported for potential application as catalysts, catalysts supports, adsorbents as well as nanoreactors for making new materials.1–6 Mesoporous silica has a porous texture which is uniform in size (2–10 nm range) and arrangement (hexagonal, lamella, and framework). Fig. 1 shows the schematic drawing of the liquid–cystal templating mechanism. Hexagonal arrays of cylindrical micelles are formed in the solution, and silicate species occupy the spaces between the cylinders. The targeted mesoporous structures are, thus, obtained by burning out of organic templates. The surface of the mesoporous silicates can be modified with different groups,7–9 by which the surface functionality can be greatly adjusted and the textural properties changed.3
 | ||
| Fig. 1 Synthesis mechanism of mesoporous silica. | ||
The SBA-15 is new nanoporous silica with a hexagonal structure, large pore size , high surface area, high thermal stability and is also diffusion free due to the thicker pore walls and larger pore size respectively. This can be prepared by using commercially available triblock copolymer pluronic P123 as a structure directing agent.10 Integration of acidic functional groups (e.g., SO3H) into SBA-15 has been explored to produce promising solid acids. Surface functionalization with sulfonic acid groups was carried out according to the method described in the literature.11 The preparation strategy for the nanoreactor SBA-15-Pr–SO3H is shown in Fig. 2.
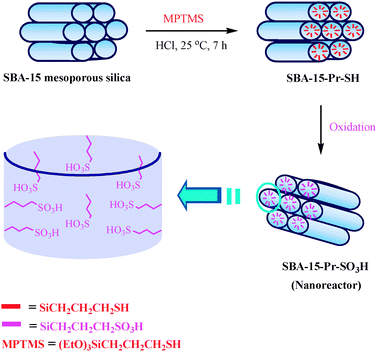 | ||
| Fig. 2 Schematic representation for the preparation of nanoreactor SBA-15-Pr–SO3H. | ||
The catalytic activity of the sulfonic acid-functionalized mesoporous silica materials is controlled by location and distribution of the acidic sites and solvent interactions.12 While several types of solid sulfonic acids, based on ordered mesoporous silicas, have been created in recent years,13 there have been only few reports about their applications as catalysts in chemical transformations.14–16 Moreover, to the best of our knowledge there is no report on the use of these materials as nanoreactors in the sulfoxidation reaction.
The oxidation of sulfides to sulfoxides is of significant importance in organic chemistry, both for fundamental research and for a wide range of applications. The oxidation of sulfides is the most straightforward method for the synthesis of sulfoxides. There are several reagents available for this key transformation.17–20 However, the reported methods rarely offer the ideal combination of simplicity of method, rapid and selective reactions, and high yields of products and often suffer from a lack of generality and economic applicability. As a consequence, the introduction of new methods and/or further work on technical improvements to overcome the limitations is still an important experimental challenge.
There has been considerable interest in the development of heterogeneous solid acid catalysts to avoid the use of traditional homogeneous acid catalytic systems which present serious drawbacks including hazards in handling, corrosiveness, production of toxic waste, and difficulties in separation. Among eco-compatible oxidants, hydrogen peroxide represents the ideal candidate, because it has high active oxygen content, it is environmentally friendly, readily available, and produces only water as a by-product.21 This feature has already stimulated the development of useful procedures for H2O2 oxidation. As a part of our continued activities in this area,19,22 herein we report an efficient protocol in which H2O2 has been used in the presence of propylsulfonic acid-functionalized mesoporous silica (SBA-15-Pr–SO3H) as a nanoreactor for the chemoselective oxidation of sulfides to sulfoxides with high activity. The route for the synthesis of sulfoxides is shown in Scheme 1.
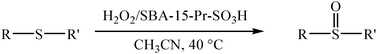 | ||
| Scheme 1 | ||
To increase accessibility of the H2O2 to the catalyst, we chose methanol or acetonitrile as solvent. To optimize the reaction conditions, we first examined the oxidation of benzyl phenyl sulfide (1 mmol) using 30% H2O2 (2 mmol) and SBA-15-Pr–SO3H (0.3 mmol%, 0.3 g) in methanol (10 mL) at 40 °C. We observed that the reaction was sluggish and only low yields (60%) of the corresponding sulfoxide were formed after 2 h. However, when a similar oxidation reaction was conducted in the presence of SBA-15-Pr–SO3H (0.6 mmol, 0.6 g), benzyl phenyl sulfoxide was efficiently formed in 98% yield within 10 min. It is noteworthy that in a blank experiment no significant oxidation was observed in the absence of SBA-15-Ph–Pr–SO3H, and only a low yield (30%) of sulfoxide was obtained in the presence of 2 equiv. of H2O2 after 6 h. Increasing the temperature to 50 °C reduced the reaction time, however, 10% of the corresponding sulfone was formed along with the sulfoxide. Control experiments showed that oxidation with less 30% H2O2 (1 equiv.) took longer (6 h) while the use of excess 30% H2O2 (3 equiv.) increased sulfone contamination, thereby reducing the selectivity of the oxidation.
After filtration of the reaction mixture, the catalyst can be recovered and recycled. Thus, after the first run, which gave the corresponding sulfoxide in 98% yield, after recovery the catalyst was subjected to a second oxidation reaction from which it gave the desired sulfoxide in 96% yield; the average chemical yield for 8 consecutive runs was 96%, which clearly demonstrates the practical recyclability of this catalyst (Fig. 3).
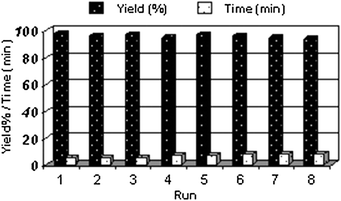 | ||
| Fig. 3 Recyclability of SBA-15-Ph-Pr-SO3H for the oxidation of benzyl phenyl sulfide. | ||
To extend the scope of the reaction and to generalize the procedure, we investigated the sulfoxidation of aryl benzyl, dibenzyl, diaryl, allyl aryl, alkyl aryl, dialkyl, cyclic and heterocyclic sulfide under optimized reaction conditions (Table 1).‡
| Entry | Sulfoxide | Yield%b (t/min) | Mp (°C)ref. |
|---|---|---|---|
| a The purified products were characterized by mp and 1H NMR spectroscopy. b Yield refers to pure isolated product. c 1,4-Dioxane was used as the solvent. | |||
| 1 |

|
98 (10) | 121–12223a |
| 2 |

|
94 (25) | 133–13423b |
| 3 |

|
93 (25) | 69–7023b |
| 4c |

|
0 (300) | — |
| 5 |

|
97 (15) | 171–17219d |
| 6 |

|
94 (5) | 72–7419d |
| 7 |

|
95 (10) | Oil19d |
| 8 |

|
96 (25) | Oil19d |
| 9 |

|
98 (15) | 110–11119d |
| 10 |
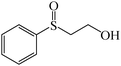
|
93 (20) | 151–15223c |
| 11 |

|
90 (17) | Oil23d |
| 12 |

|
98 (15) | 39–4023b |
| 13 |

|
97 (5) | Oil23e |
| 14 |

|
95 (15) | 29–3023d |
Inspection of the data in Table 1 clearly shows that different types sulfides were successfully converted to the corresponding sulfoxides in almost quantitative yields at 40 °C. All the reactions occurred with complete selectivity for sulfoxide formation, no overoxidation products such as sulfones were detected in the reaction mixtures. Substrates containing sensitive functional groups such as, ketone, amine, ester, carboxylic acid, alcohol and nitrile were selectively oxidized at the sulfur atom, without changes in their functional groups. Acid sensitive sulfides such as 2-[(benzylthio)methyl]furan worked well without the formation of any side products, which are normally observed either in the presence of protic or Lewis acids (Table 1, entry 6). The cyclic ring and the carbon–carbon double bond were also not affected. The presence of intact organic functional groups was confirmed by 1H NMR spectra. Similarly, linear dialkyl sulfides (entry 14) also react very quickly with excellent yield.
Surprisingly, we found that thioxanthone (Table 1, entry 4) was intact under the reaction conditions in the presence of SBA-15-Pr–SO3H, with a mesoporous structure (pore diameter ≈ 31.5 Å),16 even after 5 h. It may arise from the difficulty for both the substrate and the product to enter and escape from the mesochannels.
The proposed mechanism for the oxidation of sulfide to the corresponding sulfoxide is shown in Scheme 2. The efficiency of the oxidation can be explained by the interaction between the SBA-15-Ph-Pr-SO3H and H2O2. The OH moiety of the SBA-15-Ph–Pr–SO3H forms a strong hydrogen bond with H2O2 and increases the electrophilic ability of a peroxy oxygen atom of H2O2, and at the same time assists the leaving group (H2O) in departing from the reaction intermediate. In these reaction conditions hydrogen bonding may be assisting in controlling the chemoselectivity, because the hydrogen bond between the catalyst and the oxygen of the sulfoxides could decrease the nucleophilicity of the sulfur atom of the sulfoxides and prevent further oxidation of the sulfoxide to a sulfone. When benzyl phenyl sulfoxide was allowed to react (20 h) with this oxidation system in acetonitrile at 40 °C, the starting reactant was recovered unchanged.
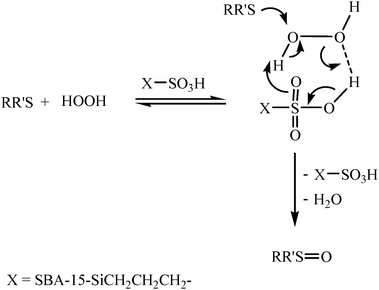 | ||
| Scheme 2 Proposed mechanism for the oxidation of sulfides to the corresponding sulfoxides with 30% H2O2 in the presence of SBA-15-Pr–SO3H. | ||
In conclusion, we demonstrate that SBA-15-Pr–SO3H is an active nanoreactor catalyst in the sulfoxidation reaction. The amphiphilic mesochannels of SBA-15 can provide a suitable accommodation for both hydrophilic hydrogen peroxide and hydrophobic sulfides. It also could be recovered and reused for several reaction cycles without considerable loss of reactivity. Furthermore, the reasonable reaction time, excellent yields, simple work-up procedure, and environmentally friendly conditions are particular merits of this method. Further investigations into the scope and synthetic applications of this nanoreactor catalyst are currently under investigation in our laboratory.
The authors acknowledge the Razi University Research Council for support of this work.
Notes and references
- A. Corma, Chem. Rev., 1997, 97, 2373 CrossRef CAS.
- C. Li, Catal. Rev., 2004, 46, 419 CrossRef CAS.
- A. Taguchi and F. Schuth, Microporous Mesoporous Mater., 2005, 77, 1 CrossRef CAS.
- X. S. Zhao, X. Y. Bao, W. P. Guo and F. Y. Lee, Mater. Today, 2006, 9, 32 CrossRef CAS.
- Y. J. Wang and F. Caruso, Chem. Mater., 2005, 17, 953 CrossRef CAS.
- (a) C. J. Liu, S. J. Li, W. Q. Pang and C. M. Che, Chem. Commun., 1997, 65 RSC; (b) C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature, 1992, 359, 710 CrossRef CAS; (c) V. S.-Y. Lin, D. R. Radu, M.-K. Han, W. Deng, S. Kuroki, B. H. Shanks and M. Pruski, J. Am. Chem. Soc., 2002, 124, 9040 CrossRef CAS.
- D. J. Macquarrie, Chem. Commun., 1996, 1961 RSC.
- X. S. Zhao and G. Q. Lu, J. Phys. Chem. B, 1998, 102, 1556 CrossRef CAS.
- D. J. Macquarrie, D. B. Jackson, J. E. G. Mdoe and J. H. Clark, New J. Chem., 1999, 23, 539 RSC.
- D. Zhao, Q. Huo, J. Feng, B. F. Chmelka and G. D. Stucky, J. Am. Chem. Soc., 1998, 120, 6024 CrossRef CAS.
- L. M. Yang, Y. J. Wang, G. S. Luo and Y. Y. Dai, Microporous Mesoporous Mater., 2005, 84, 275 CrossRef CAS.
- V. Dufaud and M. E. Davis, J. Am. Chem. Soc., 2003, 125, 9403 CrossRef CAS.
- (a) W. M. Van Rhijn, D. E. De Vos, B. F. Sels, W. D. Bossaert and P. A. Jacobs, Chem. Commun., 1998, 317 RSC; (b) I. Diaz, C. Marquez-Alvarez, F. Mohino, J. Perez-Pariente and E. Sastre, J. Catal., 2000, 193, 283 CrossRef CAS; (c) D. Margolese, J. A. Melero, S. C. Christiansen, B. F. Chmelka and G. D. Stucky, Chem. Mater., 2000, 12, 2448 CrossRef CAS; (d) K. Wilson, A. F. Lee, D. J. Macquarrie and J. H. Clark, Appl. Catal., A, 2002, 228, 127 CrossRef CAS; (e) X. Yuan, H. I. Lee, J. W. Kim, J. E. Yie and J. M. Kim, Chem. Lett., 2003, 32, 560 CrossRef; (f) K. Shimizu, E. Hayashi, T. Hatamachi, T. Kodama, T. Higuchi, A. Satsuma and Y. Kitayama, J. Catal., 2005, 231, 131 CrossRef CAS; (g) A. Karam, Y. Gu, F. Jérôme, J.-P. Doulitz and J. Barrault, Chem. Commun., 2007, 2222 RSC; (h) I. K. Mbraka, D. R. Radu, V. S.-Y. Lin and B. H. Shanks, J. Catal., 2003, 219, 329 CrossRef CAS; (i) I. Diaz, F. Mohino, J. P. Pariente and E. Sastre, Appl. Catal., A, 2003, 242, 161 CrossRef CAS; (j) J. A. Melero, R. van Grieken and G. Morales, Chem. Rev., 2006, 106, 3790 CrossRef CAS; (k) M. A. Jackson, I. K. Mbraka and B. H. Shanks, Appl. Catal., A, 2006, 310, 45.
- (a) B. Das, K. Venkateswarlu, H. Holla and M. Krishnaiah, J. Mol. Catal. A: Chem., 2006, 253, 107 CrossRef CAS; (b) M. Onaka, N. Hashimoto, Y. Kitabata and R. Yamasaki, Appl. Catal., A, 2003, 241, 307 CrossRef CAS.
- R. I. Kureshy, I. Ahmad, K. Pathak, N. H. Khan, S. H. R. Abdi and R. V. Jasra, Catal. Commun., 2009, 10, 572 CrossRef CAS.
- B. Karimi and D. Zareyee, Org. Lett., 2008, 10, 3989 CrossRef CAS.
- (a) F. G. Bordwell and P. Boutan, J. Am. Chem. Soc., 1957, 79, 717 CrossRef CAS; (b) G. W. Gokel, H. M. Gerdes and D. M. Dishong, J. Org. Chem., 1980, 45, 3634 CrossRef CAS; (c) J. M. Khurana, A. K. Panda, A. Ray and A. Gogia, Org. Prep. Proced. Int., 1996, 28, 234 CrossRef CAS; (d) K. R. Roh, K. S. Kim and Y. H. Kim, Tetrahedron Lett., 1991, 32, 793 CrossRef CAS.
- (a) T. Durst, J. Am. Chem. Soc., 1969, 91, 1034 CrossRef CAS; (b) N. J. Leonard and C. R. Johnson, J. Org. Chem., 1962, 27, 282 CrossRef CAS; (c) C. R. Johnson and J. E. Keiser, Org. Synth., 1973, 791; (d) J. Drabowicz, W. Midura and M. Kolajczyk, Synthesis, 1979, 39 CrossRef CAS.
- (a) K. Bahrami, Tetrahedron Lett., 2006, 47, 2009 CrossRef CAS; (b) M. M. Khodaei, K. Bahrami and A. Karimi, Synthesis, 2008, 1682 CrossRef CAS; (c) M. M. Khodaei, K. Bahrami and M. Shiekh Arabi, J. Sulfur Chem., 2010, 31, 83 Search PubMed; (d) K. Bahrami, M. M. Khodaei and M. Shiekh Arabi, J. Org. Chem., 2010, 75, 6208 CrossRef CAS; (e) K. Bahrami, M. M. Khodaei, B. H. Yousefi and M. Shiekh Arabi, Tetrahedron Lett., 2010, 51, 6939 CrossRef CAS.
- (a) A. Rostami and J. Akradi, Tetrahedron Lett., 2010, 51, 3501 CrossRef CAS; (b) M. H. Ali and S. Stricklin, Synth. Commun., 2006, 36, 1779 CrossRef CAS; (c) M. L. Kantam, B. Neelima, Ch. V. Reddy, M. K. Chaudhuri and S. K. Dehury, Catal. Lett., 2004, 95, 19 CrossRef CAS; (d) Y. Yuan and Y. Bian, Tetrahedron Lett., 2007, 48, 8518 CrossRef CAS; (e) S. Hussain, S. K. Bharadwaj, R. Pandey and M. K. Chaudhuri, Eur. J. Org. Chem., 2009, 3319 CrossRef CAS.
- (a) M. Colladon, A. Scarso, P. Sgarbossa, R. A. Michelin and G. Strukul, J. Am. Chem. Soc., 2007, 129, 7680 CrossRef CAS; (b) C. W. Jones, Applications of Hydrogen Peroxide and Derivatives, Royal Society of Chemistry, Cambridge, U.K., 1999 Search PubMed; (c) Catalytic Oxidations with Hydrogen Peroxide as Oxidant, ed. G. Strukul, Kluwer Academic, Dordrecht, The Netherlands, 1992 Search PubMed; (d) K. Sato, M. Hyodo, M. Aoki, X.-Q. Zheng and R. Noyori, Tetrahedron, 2001, 57, 2469 CrossRef CAS; (e) E. Dumitriu, C. Guimon, A. Cordoneanu, S. Casenave, T. Hulea, C. Chelaru, H. Martinez and V. Hulea, Catal. Today, 2001, 66, 529 CrossRef CAS.
- (a) K. Bahrami, M. M. Khodaei and I. Kavianinia, Synthesis, 2007, 547 CrossRef CAS; (b) K. Bahrami, M. M. Khodaei and F. Naali, J. Org. Chem., 2008, 73, 6835 CrossRef CAS; (c) K. Bahrami, M. M. Khodaei and Y. Tirandaz, Synthesis, 2009, 369 CrossRef CAS; (d) M. M. Khodaei, K. Bahrami and Y. Tirandaz, J. Sulfur Chem., 2009, 30, 581 Search PubMed; (e) K. Bahrami, M. M. Khodaei and A. Farrokhi, Tetrahedron, 2009, 65, 7658 CrossRef CAS; (f) K. Bahrami, M. M. Khodaei and M. Soheilizad, Synlett, 2009, 2773 CrossRef CAS; (g) K. Bahrami, M. M. Khodaei and M. Soheilizad, J. Org. Chem., 2009, 74, 9287 CrossRef CAS; (h) K. Bahrami, M. M. Khodaei and M. Soheilizad, Tetrahedron Lett., 2010, 51, 4843 CrossRef CAS; (i) K. Bahrami, M. M. Khodaei and A. Nejati, Green Chem., 2010, 12, 1237 RSC.
- (a) M. H. Ali, D. R. Leach and C. E. Schmitz, Synth. Commun., 1998, 28, 2969 CAS; (b) S. S. Kim, K. Nehru, S. S. Kim, D. W. Kim and H. C. Jung, Synthesis, 2002, 2484 CrossRef; (c) M. M. Lakouraj, M. Tajbakhsh and H. Tashakkorian, Lett. Org. Chem., 2007, 4, 75 CrossRef CAS; (d) L. Xu, Y. Z. Li, Q. S. Zhang and H. S. Zhu, Synthesis, 2004, 227 CrossRef; (e) A. Fabretti, F. Ghelfi, R. Grandi and U. M. Pagnoni, Synth. Commun., 1994, 24, 2393 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, including 1H NMR spectra for some products. See DOI: 10.1039/c1cy00053e |
| ‡ General procedure for the oxidation of sulfides: In a sealed tube equipped with a stir bar, a solution of sulfide (1 mmol) in acetonitrile (10 mL) was prepared. Aqueous 30% H2O2 (2 mmol, 0.2 mL) and SBA-15-Pr–SO3H (0.6 mmol, 0.6 g, –SO3H groups) were added and the mixture was stirred at 40 °C for the time period specified in Table 1. After the completion of the reaction, the reaction mixture was diluted with ethanol and filtered to remove the catalyst. Evaporation gave the corresponding sulfoxide as the only product. All of the products are known compounds and were easily characterized by comparison with authentic samples (1H NMR, mp). |
| This journal is © The Royal Society of Chemistry 2011 |

