Conformational polymorphism facilitates assignment of trans and cis-conformers of an α-substituted oligothiophene via IR spectroscopy†
Anatoliy N.
Sokolov
,
Joseph C.
Sumrak
and
Leonard R.
MacGillivray
*
Department of Chemistry, University of Iowa, Iowa City, IA, USA. E-mail: len-macgillivray@uiowa.edu; Fax: +1 319-335-1270; Tel: +1 319-335-0563
First published on 19th October 2009
Abstract
Conformational polymorphism is exploited as a means to assign cis–trans and trans–trans conformations of an oligothiophene backbone using solid-state IR spectroscopy.
The low processing temperature and cost of organic semiconductors has led to vast research for the use of such materials in flexible electronics.1 Understanding the structures and arrangements of organic semiconductor molecules (e.g. thiophenes, pentacene) in single crystals2 and thin films is important to develop high-performance electrical devices.1–3 In this context, changes in conformation of organic semiconductor molecules, which are often flexible (e.g. oligothiophenes), have been shown to impact electrical transport properties of solid-state materials.4 Infrared (IR) spectroscopy provides a facile way to detect changes in molecular conformation. IR spectroscopy has, for example, been used to assign the supramolecular organization of oligothiophenes on solid substrates and examine internal modes of bonding (e.g. neutral benzene versus quinonoid structure).5,6
In this report, we describe the use of IR spectroscopy to assign the conformation of the backbone of a member of a well-known class of organic semiconductor molecules, namely, a terthiophene. In particular, we have identified a change in the C–H out-of-plane stretch of a thiophene ring system that is consistent with two adjacent rings adopting either a trans–trans or rare cis–trans conformation. Using IR spectroscopy to assign conformations of oligothiophenes in solid media has been a challenge owing to difficulties to isolate individual solids that exhibit different and distinct conformations of a specific oligothiophene molecule. Weak overlapping IR bands at approximately 1440 cm−1, for example, have been reported by Hotta et al. to support two conformations of a quarterthiophene in a thin film, however, the relative amounts of each conformation in the sample was temperature dependent.7 Different conformations can also be present in the same single crystal.8,9 Our use of IR spectroscopy to assign the conformations of an oligothiophene backbone has been achieved by way of conformational polymorphism. Specifically, we describe two different polymorphs of the terthiophene 2,5″-bis(4-pyridylethynyl)-5,2′,5′,2″-terthienyl (DPTT). One polymorph exists in the achiral space group C2/c while the second exists in the chiral space group P21. The two polymorphs contain two distinct conformations of the thiophene rings, namely, the commonly observed trans–trans and the rare cis–trans conformation.8,9 A terthiophene represents the minimalist case wherein these two conformations can be seen in an oligothiophene molecule. We also use our observations to analyze spectra of two larger quarter- and sexithiophenes that exhibit the all-trans conformation.10 This is expected to assist studies that seek to determine the structure and arrangements of semiconductor molecules within solid media (e.g. thin films).10,11
DPTT was synthesized via Sonogashira cross-coupling of 2,5″-dibromo-5,2′,5′,2″-terthienyl and 4-ethynylpyridine.11 The compound was characterized via1H NMR spectroscopy. Two polymorphs of DPTT were obtained from crystallizations involving different solvent conditions.
Crystallization of DPTT via slow evaporation from a solution of toluene afforded orange needles termed phase A (achiral) (Scheme 1). The slow evaporation of a benzene solution of DPTT resulted in the formation of light yellow plates termed phase C (chiral). Each sample was characterized via single-crystal X-ray diffraction‡12 and solid-state IR spectroscopy. The phases readily interconvert upon recrystallization from the appropriate solvent.
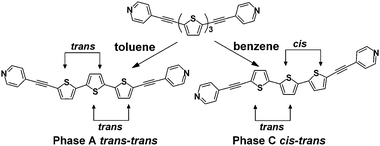 | ||
| Scheme 1 | ||
Phase A crystallizes in the achiral space group C2/c. The thiophene rings are arranged in a trans–trans conformation with an inter-ring angle of 13.2° (Fig. 1a). The all-trans conformation is consistent with the majority of reported oligothiophene single-crystal structures. The central thiophene ring is bisected by a crystallographic C2 rotation axis, which means the molecule adopts a chiral conformation with both enantiomers in the solid.
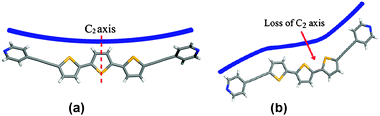 | ||
| Fig. 1 DPTT: (a) trans–trans conformation of phase A and (b) cis–trans conformation of phase C. | ||
In contrast to phase A, phase C crystallizes in a chiral crystal structure, with the space group being P21. The thiophene rings adopt a rare cis–trans conformation (Fig. 1b). We are unaware of an example of an α-substituted terthiophene that adopts a cis–trans conformation in the solid state. The thiophene ring system is relatively planar, with inter-ring angles of 7.2° (trans-rings) and 8.3° (cis-rings). Compared to phase A, the two-fold rotation axis is disrupted, with a single enantiomer of C1 symmetry being present in the solid. The spontaneous resolution of the DPTT molecules to form the chiral crystal structure presumably leads to a racemic conglomerate.13
The DPTT molecules of phase A pack in a planar, slipped-stack face-to-face orientation that exhibits modest thiophene–thiophene overlap. Specifically, half of each outer thiophene ring participates in π-stacking with a nearest-neighbour outer thiophene ring (π–π distance 3.67 Å and 3.44 Å) (Fig. 2a). In contrast, the terthienyl groups of phase C lie in 1D columns that pack herringbone to each other, with five of six hydrogen atoms of the terthiophene rings participating in C–H⋯π forces (C⋯π distances: 3.56, 3.53, 3.71, 3.78, 3.58 Å) (Fig. 2b). The two cis-related thiophene rings of each molecule point in the same direction within each column (Fig. 2c). The columns are directed along the b-axis, which coincides with the 21 screw axis. The polar arrangement of the columns is manifested in the overall chirality of the crystal. The yellow colour of phase C can be attributed to a lack of face-to-face π-stacking, as in phase A.
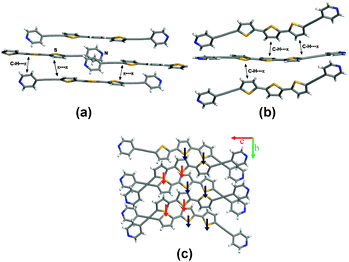 | ||
| Fig. 2 Packing of DPTT: (a) face-to-face arrangement (phase A), (b) herringbone arrangement (phase C), and (c) 1D columns of phase C highlighting the polar arrangement. | ||
The formation of the conformational polymorphs14 of DPTT has enabled us to unambiguously assign IR vibrational frequencies to the trans–trans and cis–trans conformations of the oligothiophene. In particular, phase A exhibits two strong peaks at 792.9 and 805.3 cm−1 (Fig. 3a, right arrow), which correspond to the β-hydrogens of the thiophene rings. These peaks are consistent with γC–H bends of α-substituted thiophene rings in a trans–trans conformation, which are reported as two peaks centered at ∼795 cm−1.15,16 In contrast, phase C displays three distinct bands at 790.22, 796.64, 804.14 cm−1 (Fig. 3b, right arrow). The additional bending mode can be attributed to the loss of symmetry of the terthienyl moiety that results from the cis–trans conformation of the molecule. These observations are in agreement with recent theoretical data involving a related bithiophene.17 To our knowledge, this is the first example in which the cis–trans conformation of an isolated oligothiophene has been unambiguously assigned using IR spectroscopy in the solid state. The IR stretch at approximately 1440 cm−1 (left arrows, Fig. 3a and b) reported by Hotta et al. is present in both polymorphs.7 IR data from the all-trans conformations of quarter- and sexithiophenes are in agreement with the anticipated peak positions based on the approach described by Millefiori et al.,17 which display peaks centered at 796 and 794 cm−1, respectively.10
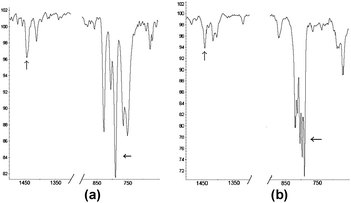 | ||
| Fig. 3 IR spectra: (a) phase A with the trans–trans form and (b) phase C with the trans–cis form of DPTT. | ||
In conclusion, we have employed conformational polymorphism to distinguish trans- and cis-conformations of an oligothiophene using IR spectroscopy. We expect these results to contribute to an improved understanding of the structures and properties of thiophene-based semiconductor solids, where conformations and structures are known to strongly influence device performance.
Notes and references
- Z. Bao and J. Locklin, Organic Field-Effect Transistors, Taylor & Francis Group, Boca Raton, FL, 2007 Search PubMed.
- V. Podzorov, V. M. Pudalov and M. E. Gershenson, Appl. Phys. Lett., 2003, 82, 1739 CrossRef CAS.
- S. C. B. Mannsfeld, A. Virkar, C. Reese, M. F. Toney and Z. Bao, Adv. Mater., 2009, 21, 2294 CrossRef CAS.
- M. A. Summers, P. R. Kemper, J. E. Bushnell, M. R. Robinson, G. C. Bazan, M. T. Bowers and S. K. Buratto, J. Am. Chem. Soc., 2003, 125, 5199 CrossRef CAS.
- P. Lang, R. Hajlaoui, F. Garnier, B. Desbat, T. Buffeteau, G. Horowitz and A. Yassar, J. Phys. Chem., 1995, 99, 5492 CrossRef CAS.
- C. Ehrendorfer and A. Karpfen, J. Phys. Chem., 1995, 99, 5341 CrossRef CAS.
- H. Muguruma, T. K. Saito and S. Hotta, Thin Solid Films, 2003, 445, 26 CrossRef CAS.
- R. Azumi, E. Mena-Osteritz, R. Boese, J. Benet-Buchholz and P. Baüerle, J. Mater. Chem., 2006, 16, 728 RSC.
- H. Muguruma, K. Kobiro and S. Hotta, Chem. Mater., 1998, 10, 1459 CrossRef CAS.
- G. Louarn, J. P. Buisson, S. Lefrant and D. Fichou, J. Phys. Chem., 1995, 99, 11399 CrossRef CAS.
- A. N. Sokolov, T. Frišcic and L. R. MacGillivray, J. Am. Chem. Soc., 2006, 128, 2806 CrossRef CAS.
- Sheldrick GM (1997) SHELXL-97: A Program for Crystal Structure Refinement (University of Göttingen, Göttingen, Germany).
- L. Pérez-García and D. B. Amabilino, Chem. Soc. Rev., 2002, 31, 342 RSC.
- H. Pan, P. Liu, Y. Li, Y. Wu, B. S. Ong, S. Zhu and G. Xu, Adv. Mater., 2007, 19, 3240 CrossRef CAS.
- M. M. Oliva, J. Casado, M. M. M. Raposo, A. M. C. Fonseca, H. Hartmann, V. Hernandez and J. T. L. Navarrete, J. Org. Chem., 2006, 71, 7509 CrossRef.
- J. Casado, R. G. Hicks, V. Hernandez, D. J. T. Myles, M. C. R. Delgado and J. T. L. Navarrete, J. Chem. Phys., 2003, 118, 1912 CrossRef CAS.
- S. Millefiori, A. Alparone and A. Millefiori, J. Heterocycl. Chem., 2000, 37, 847 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed FTIR spectra of Phase A and Phase C. CCDC 668716 (phase A) and 668717 (phase C). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/b915609g |
| ‡ Crystal Data: phase A: formula = C26H14N2S3, formula weight = 450.57, collection temperature = 293 K, monoclinic, space group = C2/c, a = 14.369(1) Å, b = 14.785(2) Å, c = 10.658(1) Å, β = 110.61(1)°, V = 2119.3(4) Å3, Z = 4, pcalc = 1.41 g cm−3, R1 = 0.039 for 1786 reflections with I > 2σ(I), Rall = 0.065 for 2435 total reflections; phase C: formula = C26H14N2S3, formula weight = 450.57, collection temperature = 293 K, monoclinic, space group = P21a = 7.464(1) Å, b = 5.675(1) Å, c = 24.501(2) Å, β = 91.02(1)°, V = 1037.7(2) Å3, Z = 2, pcalc = 1.44 g cm−3, R1 = 0.037 for 3558 reflections with I > 2σ(I), Rall = 0.057 for 4382 total reflections. The absolute structure configuration of phase C was determined by refinement of the Flack parameter, −0.08(7). IR spectra were recorded using a Nicolet 380 single beam FT-IR spectrophotometer. |
| This journal is © The Royal Society of Chemistry 2010 |
