Structure and oxide ion conductivity in Bi28Re2O49, a new bismuth rhenium oxide containing tetrahedral and octahedral Re(VII)
T. E.
Crumpton
a,
J. F. W.
Mosselmans
b and
C.
Greaves
*a
aSchool of Chemistry, University of Birmingham, Birmingham, UK B15 2TT. E-mail: c.greaves@bham.ac.uk
bCCLRC, Daresbury Laboratory, Warrington, WA4 4AD, UK, Cheshire
First published on 10th November 2004
Abstract
A new Bi–Re–O phase, Bi28Re2O49, has been synthesized and characterized. Its structure, determined by neutron powder diffraction, is a superstructure of the cubic fluorite unit cell: tetragonal, I4/m, a = 8.7216(1) Å, c = 17.4177(2) Å. The structure comprises an ordered framework of linked BiO4e trigonal bipyramids and square pyramids (e = lone pair of electrons), with discrete Re oxoanions at the origin and body-centre of the unit cell. The infra-red spectrum and Re K-edge X-ray absorption spectrum were consistent with the presence of tetrahedral ReO4− and octahedral ReO65− species in the ratio of 3 : 1. This correctly provides charge balance in the overall structure. The phase is found to display high oxide ion conductivity, which may relate to the presence of the two different oxoanion species, and possible migration of O2− ions between them.
Introduction
The substitution of small amounts of Bi in Bi2O3 by tetrahedrally coordinated M(VI) ions can lead to stable superstructures of the cubic fluorite unit cell. Tetragonal (I4/m; a ∼ 8.7 Å, c ∼ 17.3 Å) or monoclinically distorted variants have been reported for a Bi : M ratio of 14 : 1 with M = S, Cr, Mo and W.1–4 The tetragonal supercell parameters are related to the fluorite unit cell size, af, by a = (√10/2)af, c = 3af, giving a unit cell contents of 28Bi, 2M and 48O atoms and an overall formula Bi14SO24 or Bi14O20SO4. The basic structure comprises tetrahedral MO42− ions at the origin and body-centre of the unit cell; the MO4 units exhibit a degree of orientational disorder which appears to vary with M and temperature, and is linked to the transition from tetragonal (I4/m) to monoclinic (C2/m) symmetry observed for M = Mo,W.4 In an investigation of the possible co-substitution of M(VI) ions by M′(V) and M″(VII) species, we focussed on M″ = Re, since Bi3ReO8 has been reported and contains discrete ReO4− anions.5 However, to our surprise, the same superstructure was observed for Bi : Re ratios of 14 : 1, in the absence of M′(V) ions. Here we report the chemical nature and structure of this phase along with oxide ion conductivity data.Experimental
A synthetic route was used which was similar to that used for Bi14SO24: stoichiometric amounts of Bi2O3 and NH4ReO4 were intimately ground to give a Bi : Re ratio of 14 : 1, and the mixture was heated twice (with a re-grind between firings) at 800 °C for 12 h in air. X-Ray powder diffraction (XPD) data were collected on a Siemens D5000 (Ge primary beam monochromator giving Cu-Kα1, transmission mode, 8° PSD). Re-K-edge X-ray absorption spectra were recorded in transmission mode on Station 9.2 at the SRS, Daresbury Laboratory. A Si(220) double crystal monochromator was used, which was detuned by 40% to remove higher order harmonics. Analysis was performed as previously described6 using the SRS programs EXCALIB and EXBROOK for data reduction and EXCURV987 for data simulation. Time-of-flight neutron powder diffraction (NPD) data were collected at 298 K using the ultra high resolution back-scattering detectors on the HRPD diffractometer at ISIS, Rutherford Appleton Laboratory. The program GSAS8 was employed for structure refinement. A Shimadzu 8300 spectrophotometer was used to collect FTIR data. Magnetic measurements were made on a Quantum Design PPMS using AC susceptibility (10 Oersted) and DC magnetization in a field of 1000 Oersted. Pellets for conductivity measurements were prepared in a 13 mm diameter die at 7500 kg cm−3. After sintering at 750 °C for 12 h, pellet densities were 85–90% of theoretical. Silver paste was used to provide contact with the faces of the pellets and the conductivity was measured in air using a Hewlett Packard 4800A vector impedance meter with frequencies 100–5 × 105 Hz in the temperature range 400–600 °C.Results and discussion
The synthesized Bi14ReOx was bright yellow in colour, in marked contrast to the pale cream observed for substituted bismuth oxides in which the substituent has no associated colour, e.g. Bi14SO24.1 Tetrahedral ReO4− ions show no absorption in the visible region, and Bi3ReO8, with isolated ReO4− ions is, as expected, colourless.5 On the other hand, Re(VII) with octahedral (Li5ReO6, Na5ReO6)9,10 or trigonal bipyramidal (Na3ReO5)11 coordination by oxygen shows a strong yellow/orange colouration owing to charge-transfer bands. Infra-red spectroscopy, Fig. 1, revealed bands in the vicinity of 900 cm−1, characteristic of tetrahedral ReO4−, and 600–700 cm−1, which appears to be typical of octahedral ReO65− species.5 Splitting of the band at ∼900 cm−1 suggests that the ReO4− ions are significantly distorted from regular tetrahedral symmetry.5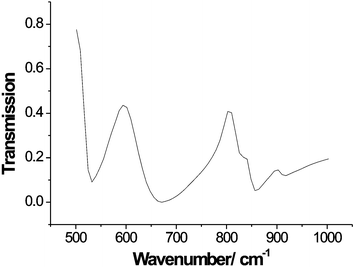 | ||
| Fig. 1 FTIR spectrum of Bi28Re2O49. | ||
XPD suggested that the product was single phase with the tetragonal (I4/m) superstructure exhibited by Bi14SO241 and Bi14CrO24.3,4 The synthesis conditions require the formation of Re(VII) rather than lower valent ions, which suggests a formula of Bi14ReO24.5 and a unit cell content of Bi28Re2O49. Evidence from infra-red spectroscopy suggests the likelihood that 75% of the Re ions are present as ReO4− ions, and 25% as ReO65− ions. The absence of reduced Re species was confirmed by magnetization measurements, which demonstrated that the phase is diamagnetic.
High resolution NPD data were collected at ambient temperature to explore the structural details of Bi28Re2O49. As a result of the orientational disorder of the tetrahedral groups in the related Bi14MO24 compounds,4 and the consequent difficulty of locating the O atoms within these groups, it was considered that realistic information concerning the orientation of the mixed oxoanions in Bi28Re2O49 would not be attainable even at low temperatures. Structure refinement therefore focussed on the basic Bi–O structural framework at 298 K. Using the room temperature structure of Bi14CrO24 (I4/m)4 as a starting model, refinement proceeded in a straightforward manner with the exclusion of the O atoms bonded to Re. As expected, subsequent attempts to locate these atoms proved difficult. However, given that 75% of the Re was thought to be present as ReO4−, a successful refinement was achieved by making the approximation that only tetrahedral species were in the structure. On this basis, the final refinement confirmed the structure, and provided a bismuth oxide framework which is essentially the same as that found in the related Bi14MO24 compounds. Although less information can be obtained for the Re oxoanions, it appears that the ReO4− ions present have an orientation very similar to that reported previously for Bi14MO24, M = Cr, Mo, W.4 As observed for these phases, the Re is displaced off the origin along [001], by ∼0.17 Å. The fitted NPD profiles are shown in Fig. 2 and the refined atomic positions and other structural information are provided in Table 1. Even after allowing displacement of Re, its isotropic temperature factor remained rather high, as is the value for O5 which is bonded to it. It is likely that these values reflect disorder associated with the presence of both ReO4− and ReO65− ions in the structure, but attempts at splitting the Re site further, to allow for these species, did not improve the refinement. Fig. 3 shows the structural framework, highlighting the presence of the Bi2O4 distorted tetrahedra (trigonal bipyramids if we include the stereochemical lone-pair of electrons [e], Bi2O4e) and square pyramids (Bi1O4e and Bi3O4e with e at the pyramid apex). Owing to the orientational disorder of the ReO4− units (and the fact that the ReO65− units have not been incorporated), the O atoms bonded to Re (O4, O5, O6 and O7) have not been included in Fig. 3. Selected bond distances and angles are given in Table 2.
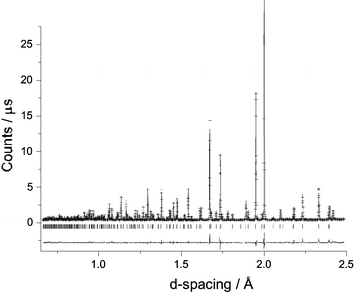 | ||
| Fig. 2 Observed (+), calculated and difference NPD profiles of Bi28Re2O49 at 298 K. The reflection positions are marked (|). | ||
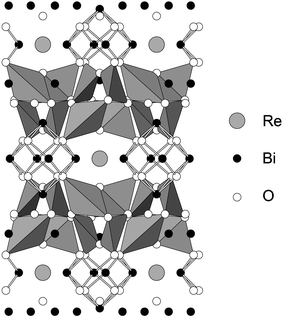 | ||
| Fig. 3 The structure of Bi28Re2O49 showing the BiO4e trigonal bipyramids and the Bi–O bonds in the BiO4e square pyramids. The O atoms bonded to Re are not shown. | ||
| Atom | Position | x | y | z | U iso × 100/Å2 | Cell occupancy |
|---|---|---|---|---|---|---|
| a I4/m; a = 8.7216(1) Å, c = 17.4177(2) Å; Rp = 0.0465, Rwp = 0.0548, Rexp = 0.0216. | ||||||
| Re | 4e | 0 | 0 | 0.0100(6) | 4(2) | 2 |
| Bi1 | 8h | 0.2107(3) | 0.4491(2) | 0 | 1.82(5) | 8 |
| Bi2 | 16i | 0.2998(2) | 0.1056(2) | 0.1711(8) | 1.66(3) | 16 |
| Bi3 | 4e | 0.5 | 0.5 | 0.1557(2) | 2.33(9) | 4 |
| O1 | 8g | 0.5 | 0 | 0.1259(2) | 2.66(6) | 8 |
| O2 | 16i | 0.0739(2) | 0.2517(3) | 0.2567(1) | 2.37(5) | 16 |
| O3 | 16i | 0.3253(3) | 0.6302(3) | 0.0780(1) | 2.16(7) | 16 |
| O4 | 16i | 0.020(2) | 0.232(2) | 0.015(2) | 0.3(2) | 2 |
| O5 | 4e | 0 | 0 | 0.1126(6) | 4.1(3) | 2 |
| O6 | 16i | 0.015(3) | 0.179(3) | 0.036(2) | 2.3(2) | 2 |
| O7 | 16i | 0.995(2) | 0.206(3) | 0.012(3) | 2.3(2) | 2 |
| Re–O4 | 2.03(2) | Bi2–O1 | 2.131(2) |
| Re–O5 | 1.79(2) | Bi2–O3 | 2.584(2) |
| Re–O6 | 1.76(3) | Bi2–O2 | 2.086(3) |
| Re–O7 | 1.84(3) | Bi2–O2 | 2.202(3) |
| O4–Re–O5 | 101.9(9) | O1–Bi2–O2 | 96.0(1) |
| O4–Re–O6 | 106.2(9) | 93.0(1) | |
| O4–Re–O7 | 97(2) | O1–Bi2–O3 | 83.7(1) |
| O5–Re–O6 | 106.2(9) | O2–Bi2–O2 | 96.7(1) |
| O5–Re–O7 | 102(1) | O2–Bi2–O3 | 79.1(1) |
| O6–Re–O7 | 99(1) | 175.8(1) | |
| Bi1–O3 | 2.221(3) [×2] | Bi3–O3 | 2.334(3) [×4] |
| 2.311(3) [×2] | |||
| O3–Bi1–O3 | 72.0(1) | O3–Bi3–O3 | 70.31(9) [×4] |
| 72.7(1) [×2] | 109.0(2) [×2] | ||
| 75.4(1) | |||
| 114.9(2) [×2] |
The Bi/O framework is seen to be essentially the same as that previously reported for the M(VI) analogous materials4 and consists of linked BiO4 polyhedra with stereochemically active lone pairs of electrons. The Re–O bonds to O5,6,7 are typical of those in tetrahedral ReO4− ions (e.g. average 1.74 Å in Bi3ReO85), whereas the Re–O4 bond is significantly longer and is similar to distances observed for octahedral ReO65− species, e.g. 1.89 Å in Li5ReO6 and Na5ReO6,8 and La3ReO8.12,13 The coordination around Bi is typical for BiO4e arrangements and the Bi–O distances are very similar to those observed in related structures: bonds of 2.2–2.3 Å for the square pyramidal Bi1O4e and Bi3O4e with the trigonal bipyramidal Bi2O4e having the equatorial bonds (2.131(2) Å and 2.086(3) Å) significantly shorter than the axial bonds (2.584(2) Å and 2.202(3) Å).
Given the difficulties in locating O atoms bonded to Re in this phase, owing to orientational disorder and the presumed presence of both ReO4− and ReO65− ions, EXAFS data were collected and compared with reference materials KReO4 (tetrahedral Re) and Li5ReO6 (octahedral Re). The coordination around Re was modelled as a single species and the calculations revealed two different Re–O distances: 1.74(2) Å [3.1(5) bonds per Re, Debye–Waller factor 0.004(1) Å2] and 2.15(2) Å [1.5(2) bonds per Re, Debye–Waller factor 0.002(1) Å2] and a fit index, R = 32.6%.6Fig. 4 shows the experimental and calculated EXAFS k3χ(k) data and their Fourier transform. Although the longer bond distance of 2.15 Å is slightly longer than expected for octahedrally coordinated Re(VII), the 1.74 Å bond is highly typical of tetrahedral ReO4− ions. The EXAFS data therefore provide good support for the structural model involving 75% ReO4− and 25% ReO65−. Based on a single Re species, this mixture of ions would result in an average Re coordination of 4 × 0.75 short (tetrahedral) bonds and 6 × 0.25 long (octahedral) bonds, i.e. 3 short and 1.5 long bonds, in excellent agreement with the calculated local model.
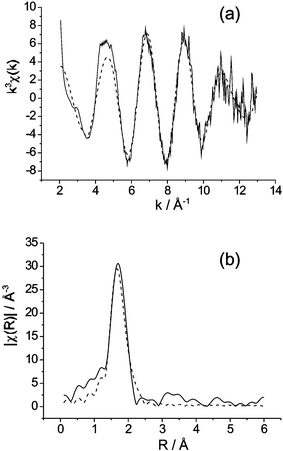 | ||
| Fig. 4 Experimental (solid line) and calculated (- - - ) Re K-edge EXAFS data for Bi28Re2O49: (a) k3 weighted EXAFS, (b) phase shifted Fourier transform of k3 weighted EXAFS. | ||
Reasonably high oxide ion conductivity has recently been reported in a bismuth oxide phase containing tetrahedral sulfate groups: Bi8SO15 or BiO11(SO4).14 In this material, a high level of disorder was apparent on the oxygen sublattice, and the conductivity in the range 400–600 °C was determined to be within an order of magnitude of that observed for the Y-stabilised δ-Bi2O3 phase, (Bi0.75Y0.25)2O3 or Bi3YO6. In Bi28Re2O49, although the bismuth oxide framework has no apparent disorder, the presence of both ReO4− and ReO65− ions in a random fashion suggests that a conduction path may exist for the support of oxide ion migration. Conductivity measurements were therefore made between 400 and 600 °C. The complex plane impedance plots could be fitted to a single semicircle, with a Warburg impedance at low frequency that was attributed to electrode/electrolyte interfacial effects. The response was similar to that observed by us for well characterised stabilised Bi2O3 oxide ion conductors (e.g. Bi3YO6), and the resistance was assigned to bulk effects. It was assumed that under the oxidising conditions used, the electronic contribution to the conductivity was negligible, as has been found for other oxide ion conductors based on Bi2O3.15Fig. 5 shows plots of log (σT/S cm−1 K) versus 1000/T (K−1) for Bi3YO616 and Bi28Re2O49, from which the Arrhenius activation energies, Ea, were calculated. Bi28Re2O49 produced a linear plot with Ea = 0.62 eV, which is slightly lower than the reported high temperature value for Bi3YO6 (Ea = 0.66 eV).16 For the temperature range studied, Bi28Re2O49 exhibits conductivity very similar to that observed for the disordered phase Bi8SO15, and is higher at low temperature: at 400 °C, the conductivities are 5.4 × 10−4 S cm−1 and 4.9 × 10−4 S cm−1, respectively, in comparison with 2.0 × 10−3 S cm−1 for Bi3YO6.16 The high conductivity, despite the high level of order within a large part of the structural framework, suggests that materials containing both tetrahedral ReO4− and octahedral ReO65− units may provide interesting future targets for highly conducting solids.
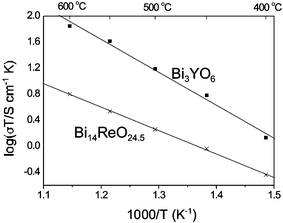 | ||
| Fig. 5 Plots of log (σT) versus 1000/T for Bi28Re2O49 compared with Bi3YO6.16 The lines are linear fits to the data. | ||
Conclusions
This study has identified a new phase with a structure closely related to that previously reported for Bi14MO241–4 materials, where M forms discrete MO42− tetrahedral groups. When Re is the substituting cation, an interesting charge balancing mechanism is found in which 25% of the ReO4− ions are replaced by ReO65− ions, and the overall composition becomes Bi14ReO24.5 or Bi28Re2O49. Although NPD was able to confirm the basic structural properties, the precise oxygen arrangement around the Re ions could not be established owing to orientational disorder of the Re oxoanions, and the small concentration of the octahedral species. EXAFS data, however, provided strong support for the presence of two distinct Re–O bond distances, and the ratio of the number of bonds agreed well with that expected for the proposed ratio of ReO4− and ReO65− ions. The phase exhibits high oxide ion conductivity which may be supported by a mechanism involving O2− transport from ReO65− to ReO4−.Acknowledgements
We thank EPSRC for the provision of financial support, and CCLRC for the provision of neutron diffraction and synchrotron facilities. We are grateful to Dr K. S. Knight for experimental assistance with the collection of NPD data.References
- M. G. Francesconi, A. L. Kirbyshire, C. Greaves, O. Richard and G. Van Tendeloo, Chem. Mater., 1998, 10, 626 CrossRef CAS.
- C. D. Ling, R. L. Withers, J. G. Thompson and S. Schmid, Acta Crystallogr., Sect. B, 1999, 55, 306 CrossRef.
- S. A. Warda, W. Pietzuch, W. Massa, U. Kesper and D. Reinen, J. Solid State Chem., 2000, 149, 209 CrossRef CAS.
- T. E. Crumpton, M. G. Francesconi and C. Greaves, J. Solid State Chem., 2003, 175, 197 CrossRef CAS.
- A. K. Cheetham and A. R. R. Smith, Acta Crystallogr., Sect. B, 1985, 41, 225 CrossRef.
- O. Helgason, J.-M. Grenache, F. J. Berry, S. Morup and F. Mosselmans, J. Phys.: Condens. Matter, 2001, 13, 10785 CrossRef CAS.
- N. Binsted, J. W. Campbell, S. J. Gurman, I. Ross and P. C. Stephenson, EXCURV98, CCLRC Daresbury Laboratory Computer Program Search PubMed.
- R. B. Von Dreele, A. C. Larson and M. Lucan, GSAS, Neutron Scattering Centre, LANL, Los Alamos, NM 87545, 1995.
- T. Betz and R. Hoppe, Z. Anorg. Allg. Chem., 1984, 512, 19 CrossRef.
- L. R. Morss, E. H. Appelman, R. R. Gerz and D. Martin-Rovet, J. Alloys Compd., 1994, 203, 289 CrossRef CAS.
- E. Vielhaber and R. Hoppe, Z. Anorg. Allg. Chem., 1992, 610, 7 CAS.
- G. Baud, J. Besse, R. Chevalier and M. Gasperon, J. Solid State Chem., 1979, 29, 267 CrossRef CAS.
- A. R. R. Smith, A. K. Cheetham and H. Fuess, Z. Anorg. Allg. Chem., 1984, 510, 46.
- T. E. Crumpton and C. Greaves, J. Mater. Chem., 2004, 14, 2433 RSC.
- P. Shuk, H.-D. Wiemhöfer, U. Guth, W. Göpel and M. Greenblatt, Solid State Ionics, 1996, 89, 179 CrossRef CAS.
- T. Takahashi and H. Iwahara, Mater. Res. Bull., 1978, 13, 1447 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2005 |
