Biofouling and antifouling
Nobuhiro Fusetani
Graduate School of Agricultural and Life Sciences, The University of Tokyo, Tokyo 113-8657, Japan
First published on 25th November 2003
Abstract
Covering: up to June 2003
Most benthic organisms produce planktonic larvae in their life cycles; larval settlement and metamorphosis are influenced by many environmental factors, especially chemical cues originating from conspecific adults, prey organisms, and substrates. On the other hand, larval settlement of other species endangers the survival of benthic organisms which therefore have antifouling defense. Marine natural products involved in biofouling and antifouling are described.
 Nobuhiro Fusetani | Nobuhiro Fusetani grew up in Tokyo, and received his B. Agric. in 1966 from the Faculty of Agriculture, University of Tokyo. After he completed his M.S. degree with Professor Yoshiro Hashimoto at the University of Tokyo, he spent two years with Professor Paul J. Scheuer at the University of Hawaii. He then took up an assistant professorship in 1971 at the Faculty of Agriculture, University of Tokyo. In 1975 he was awarded his PhD under the guidance of Professor Hashimoto, from the University of Tokyo. He was appointed Associate Professor in 1977 and has been a full Professor since 1990. From 1991 to 1996 he led a research group of 20 persons working on mechanisms of larval settlement and metamorphosis of marine organisms as Project Director of the Fusetani Biofouling Project, a 5-year project of the Exploratory Research for Advanced Technology (ERATO), Japan Science and Technology Corporation (JST). Currently his group's focus is on the discovery of useful leads from marine sponges and on chemical signals of crustaceans and fish. |
1 Introduction
Most benthic marine invertebrates have a planktonic larval stage in their life cycles; larvae spend minutes to months before metamorphosing into the adult form. Microscopic larvae are widely dispersed and transported by currents over long distances. When larvae become competent in metamorphosis (in other words, larvae attain the ability to metamorphose), perhaps when larval nervous systems are fully developed,1 they begin to swim toward the bottom and search for suitable substrates for settlement and metamorphosis.2 Settlement is defined in this review as the temporal and permanent attachment to substrates (temporal attachment is observed in larvae of mobile organisms such as abalone and sea urchins). Larvae of many benthic invertebrates attach themselves to substrates where they metamorphose into juveniles when they encounter suitable substrates. Survival of larvae/juveniles is heavily dependent on where they settle; settlement on an unfavorable substrate may lead a larva to its death.3 Understanding the mechanisms of larval settlement and metamorphosis in benthic invertebrates is important to our understanding of the marine ecosystem, population dynamics of benthic marine invertebrates and marine chemical communication.Larval settlement and metamorphosis are influenced by hydrodynamics4,5 and various factors including salinity, temperature, light, topology of substrates, larval age, and nutritional conditions of larvae.6–8 Particularly important are chemical cues which originate from conspecific adults, prey organisms, or surfaces of substrates.8,9 Larval settlement can be classified into gregarious and associate settlement,9 the former of which is induced by specific chemical cues from conspecifics. In associate settlement, larvae respond to chemical cues from food, hosts, symbionts, and specific substrates. Microbial films induce larval settlement and metamorphosis in many invertebrate species.9–12 Unfortunately, no bacterial inducing factors have been characterized, although the involvement of exopolymers has been suggested.13
In addition to natural cues, a wide array of “artificial cues”8,10,14 to larval settlement and metamorphosis are known, which include neurotransmitters, inorganic ions, hormones, and pharmacological agents that affect cellular signal transduction systems.8,9,14–18 It is believed that cues are perceived by specific receptors located on a larval sensory organ, from which signals are transmitted via nervous and cellular signal transduction systems.2,14,15
Settlement of larvae of other benthic organisms may threaten the survival of individuals of benthic invertebrates.19 Therefore, benthic invertebrates have developed various defense systems against biofouling in the course of evolution, among which chemical defense is prominent.20–22 In fact, a wide range of marine natural products showing antifouling activity have been isolated from seaweeds, sponges, soft corals, and ascidians.9,23–27 On the other hand, benthic organisms often cause economic problems by settling on ships' hulls, cooling systems of power stations, aquaculture cages, and other submersible structures. Organotin compounds including tributyltin (TBT) and tributyltin oxide (TBTO) were widely used for controlling these sessile organisms until recently, when they were found to be toxic to many marine organisms.28 Currently, the use of these compounds is either prohibited or controlled, and they will be banned in the near future. Eighteen booster biocides are used as alternative antifouling paints at the moment, but they may also pose a threat to the aquatic environment.29 Therefore, “environmentally friendly” antifoulants need to be developed urgently. Many researchers are trying to employ chemical defense systems from sessile marine organisms for this purpose.27,30,31
No reviews on biofouling or antifouling have been published previously in Natural Product Reports. John Faulkner was a pioneer natural product chemist in the field of chemical ecology, with an interest in biofouling and antifouling (Nat. Prod. Rep., 2003, 20, v).
2 Chemical cues to larval settlement and metamorphosis
Many reports have been published on chemical cues to larval settlement and metamorphosis in various invertebrate species (see, comprehensive reviews9,10,23–26,32). However, very few natural cues have been fully characterized, which is partly due to the lack of appropriate assay methods and the difficulty in obtaining a supply of considerable amounts of larvae, but is also due to the limited involvement of natural product chemists in this field. In this section, marine natural products which induce larval settlement and metamorphosis are described in taxonomic order.2.1 Hydroids and corals
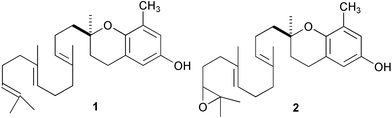
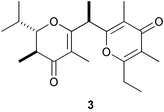
The hydroid Coryne uchidai is frequently found in colonies on brown algae of the family Sargassaceae. Nishihira33 first showed that larvae of C. uchidai stopped swimming immediately and began crawling when exposed to seawater containing small pieces of Sargassum thunbergii, S. confusum, or S. tortile. The aqueous extract of S. tortile induced similar behaviours in larvae, with metamorphosis within 2 days. Fractionation of the hexane extract of dried S. tortile led to the isolation of several chromanol diterpenes, of which δ-tocotrienol (1) and its epoxide 2 were most abundant.34 The epoxide induced larvae to metamorphose at relatively high concentrations (18.8 µg mL−1) in 72 h. It is strange that a highly lipophilic terpenoid was isolated as a natural cue, although the cue was originally thought to be hydrophilic. No further work has been done on a natural inducer in S. tortile.The colonial hydroid Hydractinia echinata is found on shells of gastropods inhabited by hermit crabs. Bacteria of the genus Pseudoaltermonas isolated from the gastropod shells induced larval settlement and metamorphosis; surface-associated macromolecules, e.g., hydrophobic proteins or lipopolysaccharides, are believed to be inducers.35 A wide range of chemicals has been reported to induce larval metamorphosis, including Cs+, Li+, K+, NH4+, phorbol esters, diacylglycerols, and GLWamides (a family of neuroactive oligopeptides possessing the C-terminal sequence of Gly-Leu-Phe found in Cnidaria).16,35–37 Based on experiments with these “artificial inducers”, Leitz16 proposed a hypothetical model for larval metamorphosis in H. echinata; the binding of the bacterial inducer to receptors causes depolarization of the sensory cells through the activation of a PI/PKC signal transduction cascade, followed by the release of a neuropeptide such as metamorphosin A (pGlu-Gln-Pro-Gly-Leu-TrpNH2)38 which transmits an internal signal triggering and synchronizing metamorphosis. Perhaps a similar mechanism of larval metamorphosis is involved in the upside-down jellyfish Cassiopea spp. whose larval settlement and metamorphosis are also triggered by bacterial films.35,37 In contrast, larval settlement of the tropical species C. xamachana is induced by a 5.8 kDa peptide rich in proline and glycine produced by bacterial decomposition of mangrove leaves of Rhizophora mangle, though it was effective only at a high concentration (86 µM).39,40
Larvae of the stony coral Agaricia humilis settle and metamorphose in response to a highly sulfated lipoglycosaminoglycan containing repeating β (1,4)-linked N-acetyllactosamine sulfate units with aliphatic side chains present on the surface of crustose coralline red algae such as Hydrolithon boergesenii.41–43 A bacterial species of Pseudoalteromas isolated from the crustose coralline red alga H. onkodes induced larval metamorphosis in the coral Acropora willisae,44 but no chemical characterization of the cue was made.
2.2 Polychaete worms
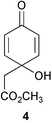
Marine polychaete worms often settle gregariously and form colonies, sometimes producing large mounds, which is common for tube-living worms. Larvae of these species are known to settle on the sand tubes of adult conspecifics. Phragmatopoma californica, commonly found along the coast of California, forms reefs of amassed sand tubes, and its larvae are induced to settle and metamorphose by the organic extract of natural sand tubes.45 An active fraction of the extract contained a mixture of fatty acids ranging from C14 to C22, in which eicosapentaenoic (20:5), palmitic (16:0), and palmitoleic acids (16:1) were predominant. In fact, palmitoleic, linoleic (18:2), arachidonic (20:4), and eicosapentaenoic acids induced larval settlement, though low percentages of metamorphosis.46 However, this result has been challenged by Morse and coworkers.47,48 These fatty acids are likely to act as “juvenile hormone (JH)-active compounds” as suggested by Biggers and Laufer; methyl farnesoate is an endogeneous JH-active compound in a marine annelid Capitella sp. I, and fatty acids such as linoleic acid and eicosatrienoic acid induced larval settlement and metamorphosis in this worm.49,50 Interestingly, vallartanone A (3), an antifeeding polypropionate metabolite of the Mexican pulmonate Siphonaria maura, was as effective as palmitoleic acid in the larval settlement assay in P. californica.51Larvae of the Japanese tube worm Hydroides ezoensis were also reported to settle and metamorphose in response to the conspecific tube extract,52 from which a new monoacylglycerol, 1-(4Z,7Z,10Z,13Z,16Z,19Z)-docosahexaenoyl-n-glycerol, was obtained as an active component;53 the synthetic sample induced larval metamorphosis at 3 × 10−6 M. Again, this monoacylglycerol is likely to act on the larval signal transduction system. The tropical species H. elegans larvae are induced to metamorphose by bacterial films;54,55 however, the natural cue remains unknown, although extensive work has been carried out in Hawaii and Hong Kong (see an excellent review17 on larval metamorphosis of H. elegans as well as other polychaetes).
2.3 Molluscs
Abalone larvae settle and metamorphose preferentially on crustose red algae of the genera Lithothamnion, Lithophyllum and Hildenbrandia.56 The aqueous extract of Lithothamnion californicum induces larval settlement and metamorphosis in Halitois rufescens at very low concentrations (10−9 M). The inducers are associated with the phycobiliproteins from which active small peptides of 640–1250 Da have been separated.57,58 Since γ-aminobutyric acid (GABA) was found to induce H. rufescens metamorphosis,59 a variety of “artificial inducers” has been reported, which include K+, L-α,ω-diamino acids, L-thyroxine, and diacylglycerol.2,14,18,56In contrast, larvae of the Japanese abalone H. discus hannai are not induced to settle by GABA; they respond to extracts of the green alga Ulvella lens and conspecific mucous trails.60 Dibromomethane occurring in this alga was found to induce larval metamorphosis.61 The larval metamorphosis is also induced by conspecific mucus, diatoms, and bacterial films (for more details, see a comprehensive review18 on abalone larval metamorphosis).
Chemical cues originating from prey organisms induce larval settlement and metamorphosis in three opisthobranchs Phestilla sibogae (the coral Porites compressa),15,62,63Adalaria proxima (the bryozoan Electra pilosa),64 and Alderia modesta (the yellow-green alga Vaucheria longicaulis),65,66 as well as the queen conch Strombus gigas (the red alga Laurencia poitei),67,68 but their cues were only partially characterized. The settlement inducers for the oyster Crassostrea gigas have been suggested to originate from microbial films,6,10 while proteinaceous inducers derived from conspecific shells were proposed for C. virginica.6,10 The waterborne settlement inducers, perhaps oligopeptides with a C-terminal arginine, have been partially purified from water conditioned with C. virginica;69 a synthetic model peptide, glycylglycyl-L-arginine (GGR), induced larval settlement at 10−10 M.70 This peptide induced not only larval settlement in C. virginica but also larval settlement in the barnacle Balanus amphitrite,71 larval release in mud crabs,71 and aggregation in the mussel Mytilus edulis72 at similar concentrations, in addition to mediating much other chemical communication in marine invertebrates.71 Recently, a reason for the efficacy of GGR and related peptides as chemical signals has been proposed.73 Thus, GGR is likely either to mimic natural inducers or to be “an artificial inducer”.
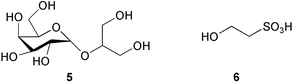
Larvae of the scallop Pecten maximus metamorphose in response to the aqueous extracts of certain marine red algae including Delesseria sanguinea, although scallops do not prey on red algae. An active component was identified as jacaranone (4) which stimulated maximum settlement at 3 × 10−6 M.74,75
Larval settlement and metamorphosis of various bivalves are also induced by bacterial films.10,76
2.4 Barnacles
Barnacle larvae show gregarious settlement, and an inducer named “arthropodin”, a surface-associated factor, was extracted from adult shells of Balanus balanoides.6,7,9 Recently, a macromolecular glycoprotein named “settlement inducing protein complex” (SIPC) was purified from Balanus amphitrite adults; this 200 kDa protein consists of three subunits of 76, 88 and 96 kDa in addition to a minor subunit of 32 kDa. The first three of these subunits were substrates for lentil lectin (LCA).77 Since LCA canceled the activity of SIPC,78 the sugar chains in the subunits appeared to be essential for activity. The immunological analysis using the antibody raised against the 76 kDa subunit demonstrated that SIPCs are present in all seven species of barnacles tested but not in other marine animals79 and that “footprint”,80 a pheromone released by barnacle larvae, is in fact SIPC.81 The field experiment showed that barnacle larvae could distinguish between conspecific SIPC and that of other species.82A waterborne settlement inducer, probably a peptide, is also released from conspecific adults,83 but only limited information on its chemical nature is available. The peptide GGR, which induced larval settlement in B. amphitrite at 10−10 M, was thought to be a candidate for the inducer;71 in fact, GGR was proved to be effective in the field.84 However, negative data were recently reported.85
The larval settlement assays using pharmacological agents and hormones led to some insights into the mechanism of larval settlement and metamorphosis in barnacles; serotonergic86 and cathecolaminergic neurons87,88 as well as the cyclic adenylase/cAMP signaling pathway89,90 are involved in larval settlement, while Ca2+, the PKC signal transduction system,91,92 and endogenous hormones such as methyl farnesoate93,94 and 20-hydroxyecdysone90,95 participate in metamorphosis. Perhaps the IP3 signaling cascade is involved, but no evidence has been obtained.90 It should be noted that Faulkner and coworkers first showed that JH and its synthetic analogues induce barnacle metamorphosis.96,97 JH is now known to mimic methyl farnesoate. Recently, detailed observation of cypris settlement behaviors has been reported;98 perhaps the receptors of cues are located on segment 4 of the antennules.99,100
2.5 Sea urchins
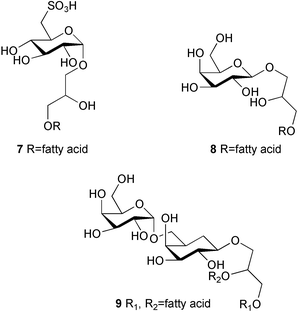
The observation that recruited juveniles of the sea urchin Holopneustes purpurascens are found primarily on the red alga Delisea pulchra led to isolation of a 1 : 1 complex of floridoside (5) and isethionic acid (6) as a larval settlement inducer from the alga.101 The synthetic complex induced larval metamorphosis at 2.5 µM, but interestingly neither floridoside nor isethionic acid alone showed the activity. The nature of the complex that these two compounds form is unclear. Quite recently, sulfoquinovosyl monoacylglycerols (SQMGs) (7) and monogalactosyl monoacylglycerols (MGMGs) (8) were isolated as the settlement inducers of the sea urchin Strongylocentrotus intermedius from the green alga Ulvella lens. Films produced by U. lens on polycarbonate plates are used for larval settlement substrates in sea urchin aquaculture in Japan.102 These two compounds induced larval settlement and metamorphosis at a concentration of 5 µg mL−1, while sulfoquinovosyl diacylglycerols (SQDGs) and digalactosyl diacylglycerols (DGDGs) (9) induced only settlement in larvae.Incidentally, these glycoglycerolipids are feeding stimulants for sea urchins.103 In addition, dibromomethane was isolated as a chemical cue for the sea urchin S. nudus from U. lens and a coralline red alga Lithophyllum sp., but its activity is much weaker than the glycerolipids.104
Coralline red algae are also known to induce larval settlement in several sea urchins.105 Unsaturated fatty acids, e.g., eicosapentaenoic acid and archidonic acid, extracted from the coralline red alga Corallina pilulifera were reported to induce larval settlement and metamorphosis in the Japanese sea urchins Pseudocentrotus depressus and Anthocidaris crassispora.106 Biofilms also induce larval metamorphosis in sea urchins, but their chemical cues remain to be elucidated.10,105
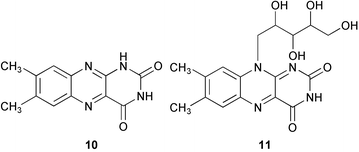
2.6 Tunicates
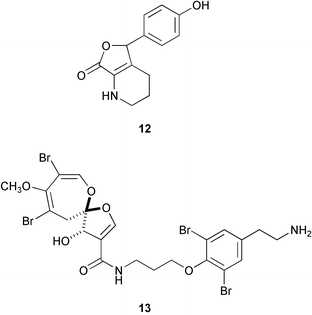
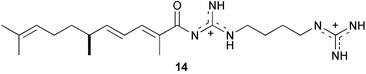
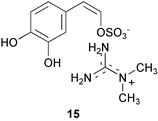
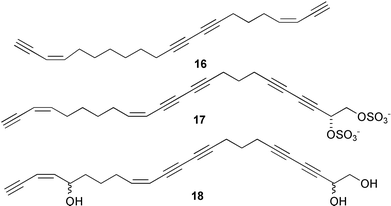
Tunicate/ascidian larvae also show gregarious settlement; larvae of some species are induced to settle and metamorphose by extracts of conspecific adult tissues.107 The Japanese edible solitary ascidian Halocynthia roretzi larvae remain swimming for a long time when larvae are kept at low densities, whereas they readily metamorphosed when kept in adult-conditioned water or at high densities (more than 50 larvae mL−1),108 which is also known for other species.109 The chemical cue was isolated from adult-conditioned water, larvae and tunic of adult individuals and identified as lumichrome (10) which may be derived from riboflavin (11) which is not active (280 µg of 10 obtained from 1000 adult ascidians).108 The purified lumichrome induced larval metamorphosis of the ascidian with EC50 10−7–10−8 M.Examination of marine invertebrate extracts for larval metamorphosis inducers in H. roretzi resulted in isolation of more than 40 compounds which include narains110 and their dimers,111 anthosamines112 [anthosamine A (12)], psammaplysin A113 (13), stellettadine A114 (14), and polyacetylenes.115 (Z)-Narain (15), a dihydroxystyrene derivative isolated from a marine sponge Jaspis sp., induced larval metamorphosis at 5 µg mL−1;110 perhaps it mimics cathecolamines.116 Psammaplysin A (13) isolated from the marine sponge Pseudoceratina purpurea and polyacetylenes from the marine sponge Callyspongiatruncata [e.g., calytetrayne (16), callyspongin B (17), and callytriol C (18)] showed not only potent larval metamorphosis inducing activity (EC50s 0.12, 0.25, 0.25, and 0.13 µg mL−1, respectively), but also inhibited cypris settlement and metamorphosis in barnacles at low concentrations (IC50s 0.27, 30, 4.1, and 0.24 µg mL−1, respectively).113,115 The true ecological roles of these compounds are not clear.
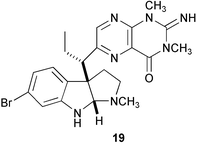
The ascidian Ciona savignyi is a group-living species, and the hydrophilic extract of its tunic was strongly inductive of conspecific larval settlement and metamorphosis. An active physostigmine derivative named urochordamine A (19) was isolated from the tunic extract; it induced larval settlement and metamorphosis at concentrations as low as 10−9 M, whereas its Z-isomer was inactive.117 It should be noted that relatively large amounts of urochordamines were found in a compound tunicate Botrylloides sp., perhaps these compounds originate from microbes. Urochordamine A also induced H. roretzi metamorphosis; probably the pteridine portion mimics lumichrome.118
Genes involved in ascidian metamorphosis which are similar to epidermal growth factor have been isolated,119–121 while NO/cGMP signaling was suggested to regulate ascidian metamorphosis.122
3 Antifouling marine natural products
A large number of antifouling compounds have been reported from marine macroorganisms, and have been reviewed by various authors.9,23–27 This section focuses on important antifoulants derived from marine organisms, together with their ecological roles and industrial application.3.1 Terpenoids
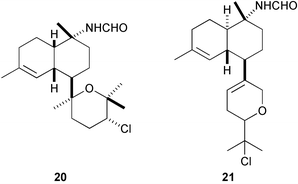
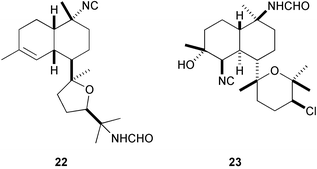
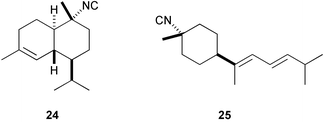
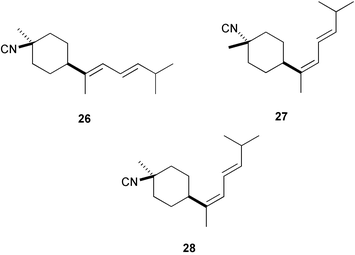
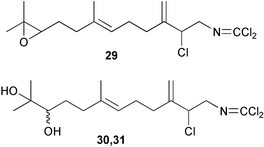
Marine sponges of the families Axinellidae and Dictyonellidae often produce sesquiterpenes and diterpenes containing isocyano, isothiocyano, thiocyano, and formamide functionalities, that exert a wide range of biological activities, e.g., antifungal, cytotoxic, ichthyotoxic, and antimalarial, whereas nudibranchs of the family Phyllidiidae sequester these terpenes from the sponges.123 However, no antifouling activity had been reported until we isolated a number of isocyanoterpenoids and related compounds mainly from the marine sponge Acanthella cavernosa and nudibranchs of the genus Phyllidia guided by a B. amphitrite assay.124 Among these terpenoids, particularly important are kalihinene X125 (20), kalihipyran B126 (21), 15-formamidokalihinene126 (22), and 10β-formamidokalihinol A127 (23), all of which inhibited the larval settlement and metamorphosis at concentrations less than 0.1 µg mL−1, while their toxicity toward the barnacle cyprids was very low (LD50s > 100 µg mL−1).A similar activity was found for 10-isocyano-4-cadinene128 (24) and isocyanotheonellin128 (25); 10-formamide-4-cadinene was as active as 24.129 Recently, isocyanotheonellin (25) and its stereoisomers 26–28 have been synthesized, indicating that neither the stereochemistry of the cyclohexane ring nor the geometry of the butadiene portion affects the activity.130,131 Three unusual sesquiterpenes containing a rare dichloromethyleneamino (carboimidic dichloride) functionality, axinyssimides A–C (29–31) were isolated from a marine sponge Axinyssa sp.;13229 inhibited cyprid settlement with an IC50 value of 1.2 µg mL−1, while 30 and 31 were more active (IC50s < 0.5 µg mL−1).
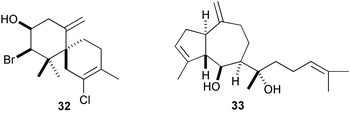
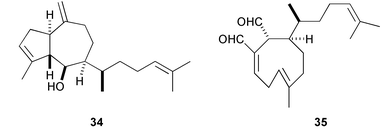
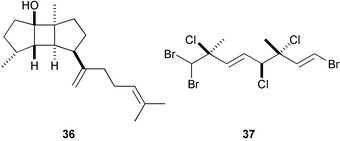
The well-known antifeeding sesquiterpene, elatol (32), derived from red algae of the genus Laurencia showed potent antifouling activity against B. amphitrite at 10 ng cm−2.133,134 Similarly, dictyol E (33), pachydictyol A (34), and dictyodial (35), antifeedant diterpenes of the brown algae Dictyota spp., are antifouling against the bryozoan Bugula neritina larvae; 33 was lethal to larvae at 5 µg mL−1, while the latter two caused abnormal development and reduced rates of growth.135,136 Dictyodial (35) also inhibited larval settlement and metamorphosis of the hydroid Eudendrium carneum. A spatane diterpene 36 from the brown alga Dilophus okamurai inhibited metamorphosis of abalone larvae at 5–10 ppb.137 A highly halogenated monoterpene 37 isolated from the Tasmanian red alga Plocamium costatum inhibited barnacle settlement at 1 µg cm−2.138
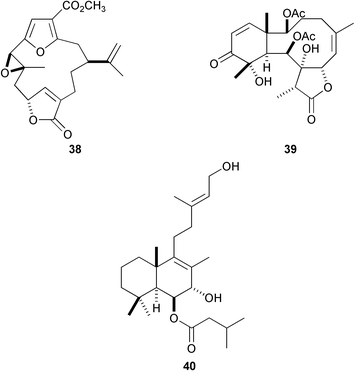
Various diterpenoids isolated from coelenterates have been reported to be antifouling against invertebrate larvae; for example, pukalide (38) from the gorgonian Leptogorgia virgulata inhibited barnacle settlement with an IC50 value of 19 ng mL−1,139,140 while renillafoulin A (39) isolated from the sea pen Renilla reniformis showed similar antifouling activity.141,142 A labdane diterpene 40 obtained from the Californian pulmonate Trimusculus reticulates inhibited the settlement of the tube worm Phragmatopoma californica at 10 µg mL−1, whereas it was lethal to the larvae at 100 µg mL−1.143
3.2 Steroids and saponins
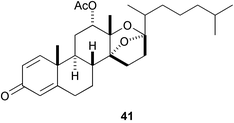
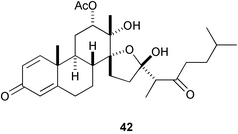
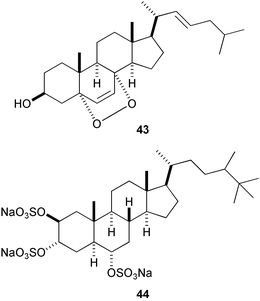
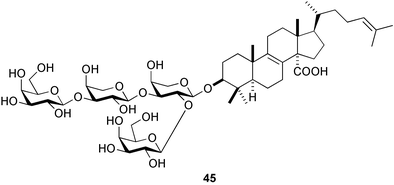
Four unprecedented D-secosteroids, isogosterone A–D [isogosterone A (41) and C (42)], isolated from a Japanese soft coral Dendronephthya sp. showed potent antifouling activity against B. amphitrite cyprids (IC50 2.2 µg mL−1),144 while four epidioxysteroids (e.g., 43) isolated from the marine sponge Acanthella cavernosa significantly prolonged larval swimming time of barnacle.145 It is noteworthy that halistanol sulfate (44) occurring in various species of marine sponges is also antifouling (IC50 5.0 µg mL−1 against B. amphitrite cyprids).146Holothurins and asterosaponins are known to be toxic to larvae of marine invertebrates.147 Recently, formoside148 (45), a triterpene glycoside derived from the Caribbean marine sponge Erylus formosus, was reported to strongly deter fouling by invertebrates and algae.149
3.3 Fatty acid-related compounds
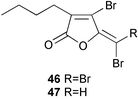
Six halogenated polyketides isolated from the Australian red alga Delisea pulchra which is free from fouling by macroorganisms showed potent antifouling activity, among which furanones 46 and 47 were most potent in B. amphitrite cyprid assay (EC50 20 ng mL−1), 20 times more active than CuSO4.150 The furanones also inhibited the settlement and growth of algal gametes as well as the growth of bacteria. It has been demonstrated that the furanones inhibit formation of bacterial films by inhibiting quorum sensing through accelerated LuxR turnover.151–153 However, it is not known how they inhibit the barnacle settlement.It is rather surprising that 1-O-palmityl-sn-glycero-3-phosphocholine (lyso-PAF) which was extracted from the marine sponge Crella incrustans was significantly antifouling against marine invertebrate larvae (0.26 µg cm−2 against B. neritina larvae).154 As mentioned above, polyacetylenes isolated from the marine sponge C. truncata were also antifouling against barnacle larvae; for example, callyspongin B (17) and callytriol C (18) are as active as CuSO4.115
3.4 Bromotyrosine derivatives
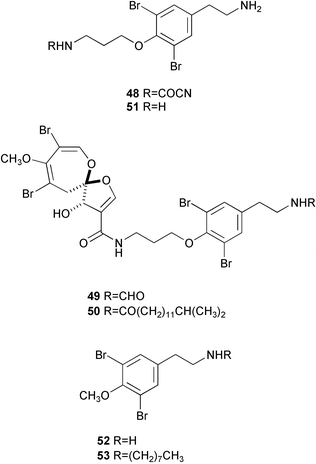
Bromotyrosine-derived metabolites are often encountered in marine sponges of the families Aplysinidae and Pseudoceratinidae, particularly Pseudoceratina (= Psammaplysilla) purpurea. Among six antifouling bromotyrosines isolated from P. purpurea collected in various locations in Japan, particularly interesting is ceratinamine155 (48) which contains a cyanoformamide functionality, unprecedented in natural products. It showed potent antifouling activity against barnacle larvae with an EC50 value of 5.0 µg mL−1. Ceratinamides A (49) and B (50), and psammaplysin A (13) showed more potent activity with EC50 values of 0.10, 2.40, and 0.27 µg mL−1, respectively.113 Recently, moroka'iamine (51) and its analogues were synthesized and evaluated for their antifouling activity against barnacle larvae.156 It is significant that such simple analogues as 52 and 53 were quite active with EC50 values of 70 and 8 ng mL−1, respectively, while they were less toxic (LD50s 200 and 30 ng mL−1, respectively). Compound 52 was isolated from the ascidian Eudistoma sp.1573.5 Heterocyclic compounds
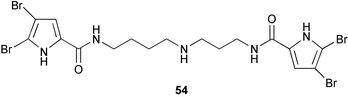
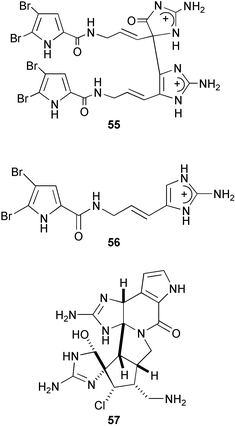
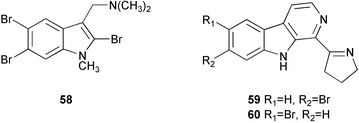
Two bromopyrrole derivatives, pseudoceratidine158 (54), a spermidine diacylated with dibromopyrrole-2-carboxylic acid, isolated from P. purpurea, and mauritiamine159 (55), an oroidin dimer from the marine sponge Agelas mauritiana showed similar antifouling activity (EC50 8.0 and 15.0 µg mL−1, respectively); oroidin (56) itself is as active as mauritiamine. Styloguanidine160 (57), an unusual phakellin-type alkaloid isolated as a chitinase inhibitor from the Yap sponge Stylotella aurantium, was later found to be antifouling against barnacle larvae, obviously affecting their molting cycle.2,5,6-Tribromo-1-methylgramine161 (58), originally isolated from the Californian bryozoan Zoobotryon verticillatum as an antibacterial constituent, was reisolated as an antifouling substance from the Japanese species Z. pellucidum.162 It showed remarkable antifouling activity, nearly 10 times more active than TBTO, whereas it was 10 times less toxic to barnacle larvae than TBTO. It is likely that 58 exerts antifouling activity by inhibiting serotonergic neurons.86 Eudistomines G (59) and H (60), β-carboline alkaloids from the Caribbean tunicate Eudistoma olivaceum,163 inhibited larval settlement of B. neritina at concentrations less than 2.2 µg cm−2.164,165
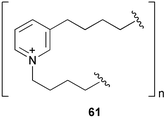
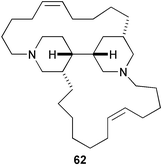
3-Alkylpyridinium derivatives are an emerging class of sponge metabolites showing a variety of bioactivities.166 Polymeric 3-alkylpyridinium salts (poly-APS)167 (61), a mixture of the 29-mer (5520 Da) and 99-mer (18,900 Da), isolated from the Mediterranean sponge Reniera sarai inhibited larval settlement of barnacles with an EC50 value of 0.27 µg mL−1.168 Perhaps poly-APS induces antifouling activity by inhibiting acetylcholine esterase in barnacle larvae.169,170 Its toxicity to barnacle nauplii is relatively low (LC50 30 µg mL−1). Strangely, haliclonacyclamine A171 (62), a major cyclic bis-(3-alkylpiperidine) isolated from a tropical sponge Haliclona sp., induced larval settlement of the ascidian Herdmania curvata, but inhibited its metamorphosis.172 It promoted larval settlement and early metamorphosis, but subsequently inhibited completion of metamorphosis.
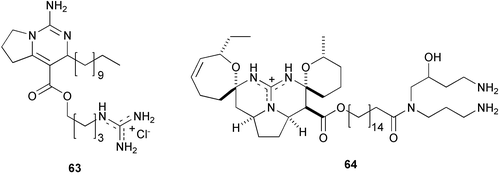
Crambe crambe, a highly successful Mediterranean sponge in chemical defense,173 contains a number of guanidine alkaloids named crambescins174,175 [crambescin A (63)] and crambescidins176,177 [crambescidin 800 (64)] which are thought to be responsible for defense against predation and macrofouling as well as for space competition.173 In fact, the alkaloid-rich fraction inhibited the settlement of B. neritina larvae,178 probably disrupting Ca2+-mediated signal transduction, since crambescidin 816 is a potent blocker of Ca2+ channels.177
3.6 Ecological relevance
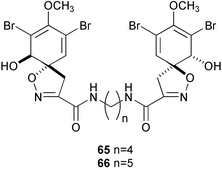
Although numbers of antifouling compounds have been isolated from various marine organisms, those which are ecologically relevant are very limited. Faulkner and coworkers first showed that antifouling bromotyrosine derivatives, aerothionin (65) and homoaerothionin (66), were released from the marine sponge Aplysina fistularis,179–181 thus implying their participation in the antifouling strategy of the sponge. Since crambescidins and related alkaloids are localized in spherulous cells182 as is the case for the aerothionins in A. fistularis,183 they are most likely released from the sponge. Cembranoid diterpenes were also found to be released from soft corals into surrounding waters,184 which clearly indicates their roles in chemical defense of soft corals.185,186 The pulmonate Trimusculus reticulates secretes mucus from its foot that contains the diterpene 37,143 thus implicating its role in defense.Halogenated furanones have been demonstrated to be encapsulated in the red alga Delisea pulchra within specialized gland cells that are present at the algal surface.187 Relatively large amounts of the furanones are released onto the algal surface,188 which also indicates their roles in chemical defense.
3.7 Biofilms
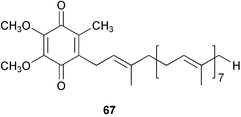
There are many reports on antifouling activity of biofilms,189,190 but no active factors have been obtained, although exopolymers were suggested to inhibit larval settlement in various species.13 An exception is ubiquinone-8 (67) which was isolated from a bacterium Alteromonas sp. separated from the marine sponge Halichondria okadai; it inhibited larval settlement of barnacle larvae at concentrations of 12.5 to 25.0 ppm.1913.8 Towards nontoxic antifoulants
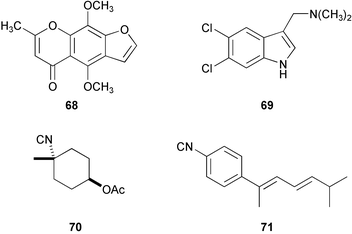
Various marine natural product antifoulants have been tested for potential industrial applications, including halogenated furanones, pukalide, and the renillafoulins. Furanones incorporated into polymers inhibited the settlement and growth of fouling organisms in the field,192 whereas the latter two compounds exhibited encouraging activity in field tests.193 Subsequently, a total of 19 furans and lactones were selected based on the structural units of pukalide and reniellafoulins and have been evaluated for antifouling activity.194 Interestingly, most analogues had narcotic activity in barnacle nauplii and cyprids. Among analogues tested, khellin (68) showed promising activity and moreover was effective in a preliminary field test.195 Perhaps poly-APS is promising as a nontoxic antifoulant judging from its low toxicity, solubility, and stability. It is also possible to synthesize a wide range of alkylpyridine polymers.196More than 500 analogues of 2,5,6-tribromo-1-methylgramine were synthesized and tested for antifouling activity, which led to the discovery of 5,6-dichlorogramine197 (69), which showed potent antifouling activity against barnacle larvae and mussels, while its toxicity was quite low (LC30 >1 µg mL−1/EC99 8 ng mL−1). After further evaluation of its antifouling activity and toxicity, a silicon-based antifouling paint containing 69 has been developed and tested in the field, and the results were very encouraging. The evaluation of the long-term efficacy is currently being conducted in Japan.
Isocyanoterpenes are also nontoxic antifoulant candidates, and more than 50 simple isocyano compounds have been synthesized and evaluated for their antifouling activity.198 Among many highly antifouling analogues, such simple compounds as 70 and 71 showed very good activity (EC50 values of 8 and 80 ng mL−1 against B. amphitrite larvae, while no toxicity was found at 100 µg mL−1). Some selective compounds will be tested in the field.
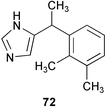
It is obvious that our knowledge of the mechanism of larval settlement and metamorphosis is applicable to development of nontoxic antifoulants.16,199 Serotonin uptake blockers and α-adrenergic antagonists have shown promising antifouling activity against barnacle larvae, based on the finding that serotonergic and adrenergic neurons are involved in barnacle settlement.200 Further experiments on several adrenoceptor antagonists disclosed that the α2-adrenoceptor antagonist medetomidine (72) is a promising nontoxic antifoulant; its LC50 (toxicity)/EC50 (antifouling activity) value was 2 × 105 against Balanus improvisus (EC50 0.1 ng mL−1).201 More importantly, this agent was very effective in panel experiments in the field.
4 References
- A. Pires, R. P. Croll and M. G. Hadfield, Biol. Bull., 2000, 198, 319 Search PubMed.
- A. N. C. Morse, Am. Sci., 1991, 79, 154 Search PubMed.
- H. L. Hunt and R. E. Scheibling, Mar. Ecol.: Prog. Ser., 1997, 155, 269 Search PubMed.
- C. A. Butman, Oceanogr. Mar. Biol. Annu. Rev., 1987, 25, 113 Search PubMed.
- A. Abelson and M. Denny, Annu. Rev. Ecol. Syst., 1997, 28, 317 Search PubMed.
- D. J. Crisp, in Chemoreception in Marine Organisms, eds. P. T. Grant and A. M. Mackie, Academic Press, London, 1974, p. 177 Search PubMed.
- D. J. Crisp, in Marine Biodeterioration: an Interdisciplinary Study, eds. J. D. Costlow and R. C. Tipper, U. S. Naval Institution, Annapolis, 1984, p. 103 Search PubMed.
- S. R. Rodríguez, F. P. Ojeda and N. C. Inestrosa, Mar. Ecol. Prog. Ser., 1993, 97, 193 Search PubMed.
- J. R. Pawlik, Oceanogr. Mar. Biol. Annu. Rev., 1992, 30, 273 Search PubMed.
- M. G. Hadfield and V. J. Paul, in Marine Chemical Ecology, eds. J. B. McClintock and B. J. Baker, CRC Press, Boca Raton, 2001, p. 431 Search PubMed.
- C. Holmström and S. Kjelleberg, Biofouling, 1994, 8, 147 Search PubMed.
- S. K. Wieczorek and C. D. Todd, Biofouling, 1998, 12, 81 Search PubMed.
- A. W. Decho, Oceanogr. Mar. Biol. Annu. Rev., 1990, 28, 73 Search PubMed.
- D. E. Morse, Bull. Mar. Sci., 1990, 46, 465 Search PubMed.
- M. G. Hadfield, Biofouling, 1998, 12, 9 Search PubMed.
- T. Leitz, Invert. Reprod. Dev., 1997, 31, 109 Search PubMed.
- P. Y. Qian, Hydrobiologia, 1999, 402, 239 Search PubMed.
- R. Roberts, J. Shellfish Res., 2001, 20, 571 Search PubMed.
- M. Wahl, Mar. Ecol. Prog. Ser., 1989, 58, 175 Search PubMed.
- S. Engel and J. R. Pawlik, Mar. Ecol. Prog. Ser., 2000, 207, 273 Search PubMed.
- D. Martín and M. J. Uriz, J. Exp. Mar. Biol. Ecol., 1993, 173, 11 CAS.
- A. R. Davis, Biofouling, 1998, 12, 305 Search PubMed.
- A. R. Davis, N. M. Targett, O. J. McConnell and C. M. Young, in Bioorganic Marine Chemistry, ed. P. J. Scheuer, Springer-Verlag, Berlin, 1989, Vol. 3, p. 86 Search PubMed.
- N. Fusetani, Curr. Org. Chem., 1997, 1, 127 Search PubMed.
- P. D. Steinberg, R. de Nys and S. Kjelleberg, in Marine Chemical Ecology, eds. J. B. McClintock and B. J. Baker, CRC Press, Boca Raton, 2001, p. 355 Search PubMed.
- P. D. Steinberg, R. de Nys and S. Kjelleberg, J. Chem. Ecol., 2002, 28, 1935 Search PubMed.
- D. Rittschof, in Marine Chemical Ecology, eds. J. B. McClintock and B. J. Baker, CRC Press, Boca Raton, 2001, p. 543 Search PubMed.
- S. M. Evans, Biofouling, 1999, 14, 117 Search PubMed.
- K. V. Thomas, Biofouling, 2001, 17, 73 Search PubMed.
- A. S. Clare, Biofouling, 1996, 9, 211 Search PubMed.
- A. S. Clare, J. Mar. Biotechnol., 1998, 6, 3 Search PubMed.
- J. R. Pawlik, in Ecological Roles of Marine Natural Products, ed. V. J. Paul, Cornell University Press, Ithaca, 1992, p. 189 Search PubMed.
- M. Nishihira, Bull. Mar. Biol. Stn. Asamushi, 1968, 13, 91 Search PubMed.
- T. Kato, A. S. Kumanireng, I. Ichinose, Y. Kitahara, Y. Kakinuma, M. Nishihira and M. Kato, Experientia, 1975, 31, 433 Search PubMed.
- W. A. Müller and T. Leitz, Can. J. Zool., 2002, 80, 1755 CrossRef.
- T. Leitz, Biofouling, 1998, 12, 173 Search PubMed.
- S. Berking, Curr. Top. Dev. Biol., 1998, 38, 81 Search PubMed.
- J. Schmich, S. Trepel and T. Leitz, Dev. Genes Evol., 1998, 208, 267 CrossRef CAS.
- J. Fleck and W. K. Fitt, J. Exp. Mar. Biol. Ecol., 1999, 234, 83 CrossRef.
- J. Fleck, W. K. Fitt and M. G. Hahn, Mar. Ecol.: Prog. Ser., 1999, 183, 115 Search PubMed.
- D. E. Morse and A. N. C. Morse, Biol. Bull., 1991, 181, 104 Search PubMed.
- A. N. C. Morse and D. E. Morse, Bioscience, 1996, 46, 254 Search PubMed.
- P. T. Raimondi and A. N. C. Morse, Ecology, 2000, 81, 3193 Search PubMed.
- A. P. Negri, N. S. Webster, R. T. Hill and A. T. Heyward, Mar. Ecol. Prog. Ser., 2001, 223, 121 Search PubMed.
- J. R. Pawlik, Mar. Biol., 1986, 91, 59 CAS.
- J. R. Pawlik and D. J. Faulkner, J. Exp. Mar. Biol. Ecol., 1986, 102, 301 CAS.
- R. A. Jensen, D. E. Morse, R. L. Petty and N. Hooker, Mar. Ecol. Prog. Ser., 1990, 67, 55 Search PubMed.
- R. A. Jensen and D. E. Morse, J. Chem. Ecol., 1990, 16, 911 Search PubMed.
- W. J. Biggers and H. Laufer, Arch. Insect Biochem. Physiol., 1996, 32, 475 CrossRef CAS.
- W. J. Biggers and H. Laufer, Biol. Bull., 1999, 196, 187 Search PubMed.
- D. C. Manker and D. J. Faulkner, J. Org. Chem., 1989, 54, 5374 CrossRef CAS.
- K. Okamoto, A. Watanabe, K. Sakata and N. Watanabe, J. Mar. Biotechnol., 1998, 6, 7 Search PubMed.
- N. Watanabe, S. Watanabe, J. Ide, Y. Watanabe, K. Sakata and K. Okamoto, J. Mar. Biotechnol., 1998, 6, 11 Search PubMed.
- C. R. C. Unabia and M. G. Hadfield, Mar. Biol., 1999, 133, 55 CrossRef.
- S. C. K. Lau, K. K. W. Mak, F. Chen and P. Y. Qian, Mar. Ecol. Prog. Ser., 2002, 226, 301 Search PubMed.
- D. E. Morse, Bull. Mar. Sci., 1985, 37, 697 Search PubMed.
- N. C. Morse, C. A. Froyd and D. E. Morse, Mar. Biol., 1984, 81, 293 CrossRef.
- A. N. C. Morse and D. E. Morse, J. Exp. Mar. Biol. Ecol., 1984, 75, 191 CrossRef CAS.
- D. E. Morse, N. Hooker, H. Duncan and L. Jensen, Science, 1979, 204, 407 CAS.
- T. Seki and H. Kan-no, Bull. Tohoku Reg. Fish Res. Lab., 1981, 43, 29 Search PubMed.
- T. Seki and K. Taniguchi, in Survival Strategies in Early Life Stages of Marine Resources, eds. Y. Watanabe, Y. Yamashita, and Y. Oozeki, A. A. Balkema, Rotterdam, 1996, p. 341 Search PubMed.
- M. G. Hadfield and J. T. Pennington, Bull. Mar. Sci., 1990, 46, 455 Search PubMed.
- M. G. Hadfield and D. Scheuer, Bull. Mar. Sci., 1985, 37, 556 Search PubMed.
- W. J. Lambert, C. D. Todd and J. D. Hardege, Invert. Biol., 1997, 116, 71 Search PubMed.
- P. J. Krug and R. K. Zimmer, J. Exp. Biol., 2000, 203, 1741 Search PubMed.
- P. J. Krug and A. E. Manzi, Biol. Bull., 1999, 197, 94 Search PubMed.
- A. A. Boettcher and N. M. Targett, J. Exp. Mar. Biol. Ecol., 1996, 196, 29 CrossRef.
- A. A. Boettcher and N. M. Targett, Biol. Bull., 1998, 194, 132 Search PubMed.
- M. N. Tamburri, R. K. Zimmer-Faust and M. L. Tamplin, Biol. Bull., 1992, 183, 327 Search PubMed.
- R. K. Zimmer-Faust and M. N. Tamburri, Limnol. Oceanogr., 1994, 39, 1075 Search PubMed.
- D. Rittschof, Am. Zool., 1993, 33, 487 Search PubMed.
- C. G. N. de Vooys, Mar. Biol., 2003, 142, 1119 Search PubMed.
- A. W. Decho, K. A. Browne and R. K. Zimmer-Faust, Limnol. Oceanogr., 1998, 43, 1410 Search PubMed.
- L. Chevolot, J. C. Cochard and J. C. Yvin, Mar. Ecol. Prog. Ser., 1991, 74, 83 Search PubMed.
- J. C. Yvin, L. Chevolot, A. M. Chevolot-Magueur and J. C. Cochard, J. Nat. Prod., 1985, 48, 814 CrossRef CAS.
- C. G. Satuito, K. Natoyama, M. Yamazaki and N. Fusetani, Fish. Sci., 1995, 61, 223 Search PubMed.
- K. Matsumura, M. Nagano and N. Fusetani, J. Exp. Zool., 1998, 281, 12 Search PubMed.
- K. Matsumura, S. Mori, M. Nagano and N. Fusetani, J. Exp. Zool., 1998, 280, 213 Search PubMed.
- K. Kato-Yoshinaga, M. Nagano, S. Mori, A. S. Clare, N. Fusetani and K. Matsumura, Comp. Biochem. Physiol., A, 2000, 125, 511 Search PubMed.
- A. S. Clare, R. K. Freet and M. McClary, Jr., J. Mar. Biol. Assoc. U.K., 1994, 74, 243 Search PubMed.
- K. Matsumura, M. Nagano, Y. Kato-Yoshinaga, M. Yamazaki, A. S. Clare and N. Fusetani, Proc. R. Soc. London, Ser. B, 1998, 265, 1825 Search PubMed.
- K. Matsumura, J. M. Hills, P. O. Thomason, J. C. Thomason and A. S. Clare, Biofouling, 2000, 16, 181 Search PubMed.
- D. Rittschof, Am. Malacol. Bull., 1985, Special Edn., 1, 111 Search PubMed.
- K. A. Browne and R. K. Zimmer, Biol. Bull., 2001, 200, 87 Search PubMed.
- A. S. Clare and M. Yamazaki, J. Mar. Biol. Assoc. U.K., 2000, 80, 945 Search PubMed.
- H. Yamamoto, A. Tachibana, S. Kawaii, K. Matsumura and N. Fusetani, J. Exp. Zool., 1996, 275, 339 Search PubMed.
- H. Yamamoto, K. Shimuzu, A. Tachibana and N. Fusetani, J. Exp. Zool., 1999, 284, 746 Search PubMed.
- K. Okano, K. Shimizu, C. G. Satuito and N. Fusetani, J. Exp. Biol., 1996, 199, 2131 Search PubMed.
- A. S. Clare, R. F. Thomas and D. Rittschof, J. Exp. Biol., 1995, 198, 655 Search PubMed.
- A. S. Clare and K. Matsumura, Biofouling, 2000, 15, 57 Search PubMed.
- H. Yamamoto, A. Tachibana, K. Matsumura and N. Fusetani, Zool. Sci., 1995, 12, 391 Search PubMed.
- E. R. Holm, M. McClary, Jr. and D. Rittschof, Mar. Ecol. Prog. Ser., 2000, 202, 153 Search PubMed.
- H. Yamamoto, T. Okino, E. Yoshimura, A. Tachibana, K. Shimizu and N. Fusetani, J. Exp. Zool., 1997, 278, 349 Search PubMed.
- P. A. Smith, A. S. Clare, H. H. Rees, M. C. Prescott, G. Wainwright and M. C. Thorndyke, Insect Biochem. Mol. Biol., 2000, 30, 885 CrossRef CAS.
- H. Yamamoto, S. Kawaii, E. Yoshimura, A. Tachibana and N. Fusetani, Zool. Sci., 1997, 14, 887 Search PubMed.
- E. D. Gomez, D. J. Faulkner, W. A. Newman and C. Ireland, Science, 1973, 179, 813 CAS.
- M. Ramenofsky, D. J. Faulkner and C. Ireland, Biochem. Biophys. Res. Commun., 1974, 60, 172 CAS.
- N. C. Lagersson and J. T. Høeg, Mar. Biol., 2002, 141, 513 Search PubMed.
- A. S. Clare and J. A. Nott, J. Mar. Biol. Assoc. U.K., 1994, 74, 967 Search PubMed.
- N. C. Lagersson, A. Garm and J. T. Høeg, J. Mar. Biol. Assoc. U.K., 2003, 83, 361 Search PubMed.
- J. E. Williamson, R. de Nys, N. Kumar, D. G. Carson and P. D. Steinberg, Biol. Bull., 2000, 198, 332 Search PubMed.
- Y. Takahashi, K. Itoh, M. Ishii, M. Suzuki and Y. Itabashi, Mar. Biol., 2002, 140, 763 Search PubMed.
- K. Sakata, K. Kato, K. Ina and Y. Machiguchi, Agric. Biol. Chem., 1989, 53, 1457 CAS.
- K. Taniguchi, K. Kurata, T. Maruzoi and M. Suzuki, Fish. Sci., 1994, 60, 795 Search PubMed.
- C. M. Pearce and R. E. Scheibling, J. Exp. Mar. Biol. Ecol., 1991, 147, 147 CrossRef.
- H. Kitamura, S. Kitahara and H. B. Koh, Mar. Biol., 1993, 115, 387 CAS.
- I. Svane, J. N. Havenhand and A. J. Jorgensen, J. Exp. Mar. Biol. Ecol., 1987, 110, 171 CrossRef.
- S. Tsukamoto, H. Kato, H. Hirota and N. Fusetani, Eur. J. Biochem., 1999, 264, 785 CrossRef CAS.
- W. F. Lynch, Am. Zool., 1961, 1, 59 Search PubMed.
- S. Tsukamoto, H. Kato, H. Hirota and N. Fusetani, Tetrahedron Lett., 1994, 35, 5873 CrossRef CAS.
- S. Tsukamoto, H. Kato, H. Hirota and N. Fusetani, Tetrahedron, 1994, 50, 13583 CrossRef CAS.
- S. Tsukamoto, H. Kato, H. Hirota and N. Fusetani, Tetrahedron, 1995, 51, 6687 CrossRef CAS.
- S. Tsukamoto, H. Kato, H. Hirota and N. Fusetani, Tetrahedron, 1996, 52, 8181 CrossRef CAS.
- S. Tsukamoto, H. Kato, H. Hirota and N. Fusetani, Tetrahedron Lett., 1996, 37, 5555 CrossRef.
- S. Tsukamoto, H. Kato, H. Hirota and N. Fusetani, J. Nat. Prod., 1997, 60, 126 CrossRef CAS.
- Y. Kimura, M. Yoshida and M. Morisawa, Dev. Biol., 2003, 258, 129 CrossRef CAS.
- S. Tsukamoto, H. Hirota, H. Kato and N. Fusetani, Tetrahedron Lett., 1993, 34, 4819 CrossRef CAS.
- S. Tsukamoto, H. Hirota, H. Kato and N. Fusetani, Experientia, 1994, 50, 680 Search PubMed.
- R. Eri, J. M. Arnold, V. F. Hinman, K. M. Green, M. K. Jones, B. M. Degnan and M. F. Lavin, Development, 1999, 126, 5809 Search PubMed.
- A. Nakayama, Y. Satou and N. Satoh, Dev. Genes Evol., 2001, 211, 184 Search PubMed.
- B. Davidson and B. J. Swalla, Dev. Genes Evol., 2001, 211, 190 Search PubMed.
- C. D. Bishop, W. R. Bates and B. P. Brandhorst, J. Exp. Zool., 2001, 289, 374 Search PubMed.
- C. W. J. Chang, Prog. Chem. Org. Nat. Prod., 2000, 80, 1 Search PubMed.
- N. Fusetani, H. Hirota, T. Okino, Y. Tomono and E. Yoshimura, J. Nat. Toxins, 1996, 5, 249 Search PubMed.
- T. Okino, E. Yoshimura, H. Hirota and N. Fusetani, Tetrahedron Lett., 1995, 36, 8637 CrossRef CAS.
- T. Okino, E. Yoshimura, H. Hirota and N. Fusetani, J. Nat. Prod., 1996, 59, 1081 CrossRef.
- H. Hirota, Y. Tomono and N. Fusetani, Tetrahedron, 1996, 52, 2359 CrossRef CAS.
- T. Okino, E. Yoshimura, H. Hirota and N. Fusetani, Tetrahedron, 1996, 52, 9447 CrossRef CAS.
- Y. Nogata, E. Yoshimura, K. Shinshima, Y. Kitano and I. Sakaguchi, Biofouling, 2003, 19(Suppl.), 193 Search PubMed.
- Y. Kitano, T. Ito, T. Suzuki, Y. Nogata, K. Shinshima, E. Yoshimura, K. Chiba, M. Tada and I. Sakaguchi, J. Chem. Soc., Perkin Trans. 1, 2002, 2251 RSC.
- Y. Kitano, A. Yokoyama, Y. Nogata, K. Shinshima, E. Yoshimura, K. Chiba, M. Tada and I. Sakaguchi, Biofouling, 2003, 19(Suppl.), 187 Search PubMed.
- H. Hirota, T. Okino, E. Yoshimura and N. Fusetani, Tetrahedron, 1998, 54, 13971 CrossRef CAS.
- R. de Nys, T. Leya, R. Maximilien, A. Afsar, P. S. R. Nair and P. D. Steinberg, Biofouling, 1996, 10, 213 Search PubMed.
- P. D. Steinberg, R. de Nys and S. Kjelleberg, Biofouling, 1998, 12, 227 Search PubMed.
- T. M. Schmitt, M. E. Hay and N. Lindquist, Ecology, 1995, 76, 107 Search PubMed.
- T. M. Schmitt, N. Lindquist and M. E. Hay, Chemoecology, 1998, 8, 125 CrossRef CAS.
- K. Taniguchi, K. Shiraishi, K. Kurata and M. Suzuki, Nippon Suisan Gakkaishi, 1989, 55, 1133 Search PubMed.
- G. M. König, A. D. Wright and R. de Nys, J. Nat. Prod., 1999, 62, 383 CrossRef.
- M. G. Missakian, B. J. Burreson and P. J. Scheuer, Tetrahedron, 1975, 31, 2513 CrossRef CAS.
- D. J. Gerhart, D. Rittschof and S. W. Mayo, J. Chem. Ecol., 1988, 14, 1905 Search PubMed.
- P. A. Keifer, K. L. Rinehart, Jr. and I. R. Hooper, J. Org. Chem., 1986, 51, 4450 CrossRef CAS.
- D. Rittschof, I. R. Hooper and J. D. Costlow, Bull. Mar. Sci., 1986, 39, 376 Search PubMed.
- D. C. Manker and D. J. Faulkner, J. Chem. Ecol., 1996, 22, 23 Search PubMed.
- Y. Tomono, H. Hirota and N. Fusetani, J. Org. Chem., 1999, 64, 2272 CrossRef CAS.
- Y. Tomono, H. Hirota and N. Fusetani, in Sponge Sciences – Multidisciplinary Perspectives, eds. Y. Watanabe and N. Fusetani, Springer-Verlag, Tokyo, 1998, p. 413 Search PubMed.
- S. Tsukamoto, H. Kato, H. Hirota and N. Fusetani, Biofouling, 1997, 11, 283 Search PubMed.
- Y. Hashimoto, Marine Toxins and Other Bioactive Marine Metabolites, Japan Scientific Societies Press, Tokyo, 1979 Search PubMed.
- M. Jaspars and P. Crews, Tetrahedron Lett., 1994, 35, 7501 CrossRef CAS.
- J. Kubanek, K. E. Whalen, S. Engel, S. R. Kelly, T. P. Henkel, W. Fenical and J. R. Pawlik, Oecologia, 2000, 131, 125 CrossRef.
- R. de Nys, P. D. Steinberg, P. Willemsen, S. A. Dworjanyn, C. L. Gabelish and R. J. King, Biofouling, 1995, 8, 259 Search PubMed.
- R. Maximilien, R. de Nys, C. Holmström, L. Gram, M. Givskov, K. Crass, S. Kjelleberg and P. D. Steinberg, Aquat. Microb. Ecol., 1998, 15, 233 Search PubMed.
- D. Ren, J. J. Sims and T. K. Wood, Environ. Microbiol., 2001, 3, 731 Search PubMed.
- M. Manefield, T. B. Rasmussen, M. Henzter, J. B. Andersen, P. Steinberg, S. Kjelleberg and M. Givskov, Microbiology, 2002, 148, 1119 Search PubMed.
- A. J. Butler, I. A. van Altena and S. J. Dunne, J. Chem. Ecol., 1996, 22, 2041 Search PubMed.
- S. Tsukamoto, H. Kato, H. Hirota and N. Fusetani, J. Org. Chem., 1996, 61, 2936 CrossRef CAS.
- R. C. Schoenfeld, S. Conova, D. Rittschof and B. Ganem, Bioorg. Med. Chem. Lett., 2002, 12, 823 CrossRef CAS.
- R. M. van Wagoner, J. Jompa, A. Tahir and C. M. Ireland, J. Nat. Prod., 1999, 62, 794 CrossRef CAS.
- S. Tsukamoto, H. Kato, H. Hirota and N. Fusetani, Tetrahedron Lett., 1996, 37, 1439 CrossRef CAS.
- S. Tsukamoto, H. Kato, H. Hirota and N. Fusetani, J. Nat. Prod., 1996, 59, 501 CrossRef CAS.
- T. Kato, Y. Shizuri, H. Izumida, A. Yokoyama and M. Endo, Tetrahedron Lett., 1995, 36, 2133 CrossRef CAS.
- A. Sato and W. Fenical, Tetrahedron Lett., 1983, 24, 481 CrossRef CAS.
- K. Kon-ya, N. Shimizu, K. Adachi and W. Miki, Fish. Sci., 1994, 60, 773 Search PubMed.
- J. Kobayashi, G. C. Harbour, J. Gilmore and K. L. Rinehart, J. Am. Chem. Soc., 1984, 106, 1526 CrossRef CAS.
- A. R. Davis and A. E. Wright, J. Chem. Ecol., 1990, 16, 1349 Search PubMed.
- A. R. Davis, Mar. Biol., 1991, 111, 375.
- K. Sepčić, J. Toxicol. Toxin Rev., 2000, 19, 139 Search PubMed.
- K. Sepčić, G. Guella, I. Mancini, F. Pietra, M. Dalla Serra, G. Menestrina, K. Tubbs, P. Macek and T. Turk, J. Nat. Prod., 1997, 60, 991 CrossRef CAS.
- M. Faimali, K. Sepčić, T. Turk and S. Geraci, Biofouling, 2003, 19, 47 Search PubMed.
- K. Sepčić, V. Marcel, A. Klaebe, T. Turk, D. Suput and D. Fournier, Biochim. Biophys. Acta, 1998, 1387, 217 CAS.
- M. Faimali, C. Falugi, L. Gallus, V. Piazza and G. Tagliafierro, Biofouling, 2003, 19(Suppl.), 213 Search PubMed.
- R. J. Clark, K. L. Field, R. D. Charan, M. J. Garson, I. M. Brereton and A. C. Willis, Tetrahedron, 1998, 54, 8811 CrossRef CAS.
- K. M. Green, B. D. Russell, R. J. Clark, M. K. Jones, M. J. Garson, G. A. Skilleter and B. M. Degnan, Mar. Biol., 2002, 140, 355 Search PubMed.
- M. A. Becerro, M. J. Uriz and X. Turon, Hydrobiologia, 1997, 355, 77 Search PubMed.
- R. G. S. Berlinck, J. C. Braekman, D. Daloze, K. Hallenga, R. Ottinger, I. Bruno and R. Riccio, Tetrahedron Lett., 1990, 31, 6531 CrossRef CAS.
- R. G. S. Berlink, J. C. Braekman, D. Daloze, I. Bruno, R. Riccio, D. Rogeau and P. Amade, J. Nat. Prod., 1992, 55, 528 CrossRef.
- E. A. Jares-Erijman, R. Sakai and K. L. Rinehart, J. Org. Chem., 1991, 56, 5712 CrossRef CAS.
- R. G. S. Berlinck, J. C. Braekman, D. Daloze, I. Bruno, R. Riccio, S. Ferri, S. Spampinato and E. Speroni, J. Nat. Prod., 1993, 56, 1007 CrossRef CAS.
- M. A. Becerro, X. Turon and M. J. Uriz, J. Chem. Ecol., 1997, 23, 1527 Search PubMed.
- J. E. Thompson, R. P. Walker and D. J. Faulkner, Mar. Biol., 1985, 88, 11 CAS.
- J. E. Thompson, Mar. Biol., 1985, 88, 23 CAS.
- R. P. Walker, J. E. Thompson and D. J. Faulkner, Mar. Biol., 1985, 88, 27 CAS.
- M. J. Uriz, M. A. Becerro, J. M. Tur and X. Turon, Mar. Biol., 1996, 124, 583 CAS.
- J. E. Thompson, K. D. Barrow and D. J. Faulkner, Acta Zool., 1984, 64, 199.
- C. Coll, B. F. Bowden, D. M. Tapiolas and W. C. Dunlap, J. Exp. Mar. Biol. Ecol., 1982, 60, 293 CrossRef.
- J. C. Coll, Chem. Rev., 1992, 90, 613 CrossRef.
- M. Maida, A. R. Carroll and J. C. Coll, J. Chem. Ecol., 1993, 19, 2285 Search PubMed.
- S. A. Dworjanyn, R. de Nys and P. D. Steinberg, Mar. Biol., 1999, 133, 727 CrossRef CAS.
- R. de Nys, S. A. Dworjanyn and P. D. Steinberg, Mar. Ecol. Prog. Ser., 1998, 162, 79 Search PubMed.
- E. Armstrong, K. G. Boyd and J. G. Burgess, Biotechnol. Annu. Rev., 2000, 6, 221 Search PubMed.
- C. Holmström, S. Egan, A. Franks, S. McCloy and S. Kjelleberg, FEMS Microbiol. Ecol., 2002, 41, 47 CrossRef CAS.
- K. Kon-ya, N. Shimizu, N. Otaki, A. Yokoyama, K. Adachi and W. Miki, Experientia, 1995, 51, 153 Search PubMed.
- R. de Nys and P. D. Steinberg, Curr. Opin. Biotechnol., 2002, 13, 244 CrossRef CAS.
- R. R. Price, M. Patchan, A. Clare, D. Rittschof and J. Bonaventura, Biofouling, 1992, 6, 207 Search PubMed.
- A. S. Clare, D. Rittschof, D. J. Gerhart, I. R. Hooper and J. Bonaventura, Mar. Biotechnol., 1999, 1, 427 CAS.
- A. S. Clare, D. Rittschof, R. R. Price and D. J. Gerhart, in Biodeterioration and Biodegradation, eds. A. Bousher and R. G. J. Edyvean, Institution of Chemical Engineers, Rugby, Vol. 9, 1995, p. 573 Search PubMed.
- A. Kaiser, X. Billot, A. Gateau-Olesker, C. Marazano and B. C. Das, J. Am. Chem. Soc., 1998, 120, 8026 CrossRef CAS.
- K. Kon-ya, N. Shimizu, W. Miki and M. Endo, Biosci. Biotechnol. Biochem., 1994, 58, 2178 CAS.
- Japan Science Technology Corporation, JP Tokukai 2002-370907.
- D. Rittschof, C. H. Lai, L. M. Kok and S. L. M. Teo, Biofouling, 2003, 19, 207 Search PubMed.
- H. Yamamoto, C. G. Satuito, M. Yamazaki, K. Natoyama, A. Tachibana and N. Fusetani, Biofouling, 1998, 13, 69 Search PubMed.
- M. Dahlström, L. G. E. Mårtensson, P. R. Jonsson, T. Arnbrant and H. Elwing, Biofouling, 2000, 16, 191 Search PubMed.
| This journal is © The Royal Society of Chemistry 2004 |