Stereoselective Lewis base catalyzed formal 1,3-dipolar cycloaddition of azomethine imines with mixed anhydrides†
Abstract
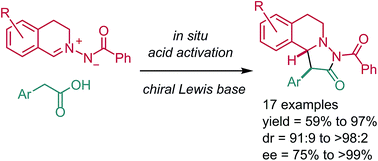
Stereoselective synthesis of pyrazolidinones via dipolar cycloaddition of azomethine imines with active esters under Lewis base catalysis is presented. The active esters are readily generated in situ from the corresponding acids. Products, which are obtained with excellent diastereocontrol and high enantioselectivity, contain along with the pyrazolidinone core also the tetrahydroisoquinoline structural motif. Theoretical studies give insight into the mechanism of the formal cycloaddition reaction.

 Please wait while we load your content...
Please wait while we load your content...