Influence of mixed CTABr–C16E20 nanoparticles on relative counterion binding constants in aqueous solutions of inert salts (2-NaOC6H4CO2Na and NaBr): kinetic and rheometric study
Abstract
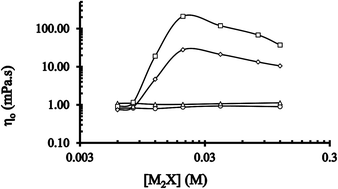
A semi-empirical kinetic technique has been used to obtain the ratios of pure CTABr (cationic nanoparticle) and mixed CTABr–C16E20 (cationic–nonionic nanoparticle) micellar binding constants of counterions X and Br, KX/KBr (=KBrX or RBrX) for X = salicylate ion. The values of KBrX or RBrX for the salicylate ion remain typically independent of the concentration of pure micelles, [CTABr]T (0.006–0.015 M). These values are also independent of mixed [CTABr]T–[C16E20]T micelles under similar conditions. The presence of C16E20 decreases the average values of KBrX or RBrX from 42 to 16 for salicylate ions. Increase in concentration of cationic and cationic–nonionic nanoparticles at a fixed amount of CTABr reveals the monotonic increase in the value of kobs, which was explained using the PPM model together with an empirical equation. Rheological characteristics of 0.015 M CTABr solutions in the presence of disodium salicylate indirectly show the presence of branched elongated nanoparticles at 25 °C and 35 °C and [C16E20]T = 0. Contrarily, such findings reveal the existence of spherical micelles at 25 °C and 35 °C in the presence of ≥0.006 M [C16E20]. The values of KBrX or RBrX and rheological observations clearly demonstrate that 0.015 M CTABr/M2X/H2O (M2X = disodium salicylate, 2-NaOC6H4CO2Na) solutions contain wormlike and spherical micelles when KBrX or RBrX values are ≈42 and 16, respectively.

 Please wait while we load your content...
Please wait while we load your content...