Abstract
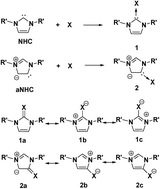
N-heterocyclic carbenes (NHC) and abnormal NHCs (aNHC) form the stable adducts, 1 and 2, with X (CH2, SiH2, NH, PH, O, S). This series of compounds has been investigated computationally, together with their H-shifted isomers, 3 and 4, which are substituted aNHC and NHC, respectively. The relative stability of the (substituted) abnormal carbenes (3) with respect to 4 does not depend on the type of substituent. The stability of the betaine forms, 1 and 2 (with respect to 4), is related to the electronegativity of the heteroatom in X, however the second and third row heteroatoms exert different effects on stabilization. NRT analysis revealed that for 1 and 2, the double bonded contributions are higher for the second than for the third row elements, which, however, have larger ionic contributions, in agreement with their large pz orbital contribution in the π-type HOMO. The Si compounds exhibit trans-bent structures in their formal double bond with large s-orbital contribution, and are consequently highly ylidic. In the case of nitrogen and phosphorus compounds, the energies of the two H-shifted forms (2 and 4) are close to each other, giving rise to a possible tautomeric equilibrium, which can be fine-tuned by proper substituents. While for the NHCs (3) and aNHCs (4), significant NICS aromaticity was obtained, the zwitterionic compounds (1 and 2), in particular those with second row substituents having a high double bond character, exhibit a reduced aromaticity.

 Please wait while we load your content...
Please wait while we load your content...