Synthesis, in vitro evaluation and DNA interaction studies of N-allyl naphthalimide analogues as anticancer agents†
Abstract
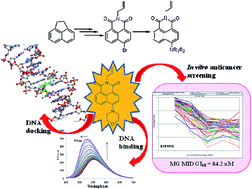
A novel series of 2-allyl-6-substituted-benzo[de]isoquinoline-1,3-diones has been synthesized and evaluated against 60 human tumor cell lines for their in vitro antitumor activities. Compound 6b proved to be the most active member at a single dose concentration of 10 μM and broad spectrum of antitumor activity with GI50, TGI and LC50 values of 84.2 nM, 27.6 μM and 89.3 μM respectively at five dose concentration levels. The DNA binding properties of this compound has been investigated by a UV-Vis and fluorescence spectrophotometer as well as thermal denaturation experiments. Molecular docking studies of compound 6b have also supported the corresponding biological data.

 Please wait while we load your content...
Please wait while we load your content...