Ready access to end-functional polystyrenes via a combination of ARGET ATRP and thiol–ene chemistry†
Abstract
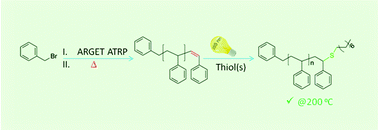
We describe a simple and facile method for quantitatively converting bromine end-groups of well-defined polystyrene (PS, Mn,SEC = 4000 Da, Đ = 1.08) prepared by activators regenerated by electron transfer (ARGET) atom transfer radical polymerization (ATRP) into terminal alkenes by heating at 100 °C in dimethylformamide (DMF) without additional reagents. Subsequently, a facile quantitative post-functionalization of the terminal double bonds to various end functional polymers was performed via light-induced thiol–ene reactions. The quantitative end-group modifications as well as their thermal stability were assessed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-ToF-MS), nuclear magnetic resonance (1H NMR) spectroscopy and size-exclusion chromatography (SEC), evidencing the generated functional polystyrenes to be highly stable up to 200 °C for extended periods of time (24 h).

 Please wait while we load your content...
Please wait while we load your content...