Lipoates as building blocks of sulfur-containing branched macromolecules†
Abstract
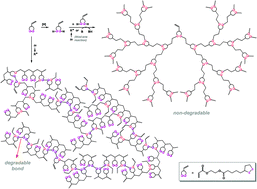
The radical copolymerization of cyclic disulfide-type monomers (ethyl lipoate or lipoic acid) with ethyl acrylate (1 : 1 molar feed ratio) yielded copolymers containing a significant fraction of backbone disulfide groups, which were degradable upon reduction. A compound containing two types of radically polymerizable groups (cyclic disulfide (1,2-dithiolane) and vinyl), 2-acryloyloxyethyl lipoate (AOELp), was synthesized and demonstrated to be a useful precursor for highly branched sulfur-containing polymers. Under radical polymerization conditions, AOELp served as a crosslinker, but up to moderate to high conversions and prior to gelation, soluble highly branched polymers were produced containing reductively degradable disulfide moieties, originating from the attack of a sulfur-centered radical on a 1,2-dithiolane group from AOELp or the polymer (pendant group), or the coupling of two sulfur-centered radicals. Alternatively, the reduction of AOELp afforded the corresponding dithiol acrylate (an AB2-type monomer), which participated in radical or ionic step-growth thiol–ene reactions, yielding highly branched reductively non-degradable polymers with thioether-type sulfur atoms in the backbones.

 Please wait while we load your content...
Please wait while we load your content...