Fibril-shaped aggregates of doxorubicin with poly-l-lysine and its derivative†
Abstract
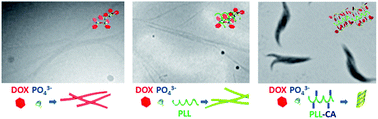
Complex formation between polymers and organic molecules is an interesting topic in polymer physics. Compared to the influence of small molecules on polymer assembly, there are fewer examples demonstrating the effect of polymers on the supramolecular structures formed by organic molecules. In this paper, we first prove that doxorubicin (DOX), a common anti-tumour drug, assembles into fibril-like aggregates in phosphate buffer (pH 7.4) and then show that the assembly of DOX was influenced by the complexation with an amphiphilic poly (amino acid) derivative, i.e. cholate-grafted poly-L-lysine (PLL-CA). With PLL-CA, the DOX fibrils converted to helix structured nano-spindles, whilst the presence of PLL led to minor change on the morphology of PLL/DOX complex compared to the DOX aggregates, which is attributed to the amplitude of intermolecular interactions. As a DNA intercalating agent, the aggregation of DOX on its biofunctionality was also investigated, showing that the formation of fibril assemblies was unfavourable for the cellular internalization of DOX and caused lower cytotoxicity to DOX resistance MCF-7 cells, whereas the polymer/DOX complexes gained an improved cell uptake on the MCF-7/ADR cell line due to an enhanced electrostatic interaction between the complexes and the cell membrane.

 Please wait while we load your content...
Please wait while we load your content...