Three-step synthesis of 2,5,7-trisubstituted indoles from N-acetyl-2,4,6-trichloroaniline using Pd-catalyzed site-selective cross-coupling†
Abstract
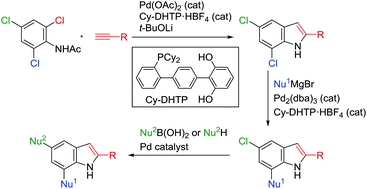
We report a facile three-step synthesis of 2,5,7-trisubstituted indoles from N-acetyl-2,4,6-trichloroaniline, with the first step featuring Pd/dihydroxyterphenylphosphine (DHTP)-catalyzed ortho-selective Sonogashira coupling followed by cyclization to afford 2-substituted 5,7-dichloroindoles. Subsequent introduction of aryl or alkenyl groups at the C7 position was achieved by Pd/DHTP-catalyzed site-selective Kumada–Tamao–Corriu coupling, with further substitution of the chlorine at the C5 position (Suzuki–Miyaura coupling or Buchwald–Hartwig amination) affording 2,5,7-trisubstituted indoles.


 Please wait while we load your content...
Please wait while we load your content...