Computationally guided discovery of a reactive, hydrophilic trans-5-oxocene dienophile for bioorthogonal labeling†
Abstract
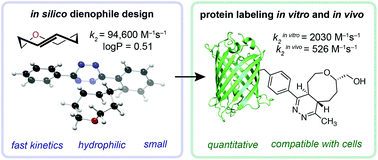
The use of organic chemistry principles and prediction techniques has enabled the development of new bioorthogonal reactions. As this “toolbox” expands to include new reaction manifolds and orthogonal reaction pairings, the continued development of existing reactions remains an important objective. This is particularly important in cellular imaging, where non-specific background fluorescence has been linked to the hydrophobicity of the bioorthogonal moiety. Here we report that trans-5-oxocene (oxoTCO) displays enhanced reactivity and hydrophilicity compared to trans-cyclooctene (TCO) in the tetrazine ligation reaction. Aided by ab initio calculations we show that the insertion of a single oxygen atom into the trans-cyclooctene (TCO) ring system is sufficient to impart aqueous solubility and also results in significant rate acceleration by increasing angle strain. We demonstrate the rapid and quantitative cycloaddition of oxoTCO using a water-soluble tetrazine derivative and a protein substrate containing a site-specific genetically encoded tetrazine moiety both in vitro and in vivo. We anticipate that oxoTCO will find use in studies where hydrophilicity and fast bioconjugation kinetics are paramount.


 Please wait while we load your content...
Please wait while we load your content...