Synthesis and regioselective functionalization of perhalogenated BODIPYs†
Abstract
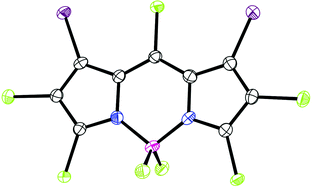
Three perhalogenated BODIPYs (1b–3b), bearing chloro and bromo groups at all carbon positions, were synthesized and characterized. The reactivity of BODIPY 3b was investigated under Stille cross-coupling reactions, and single crystal X-ray analysis was used to confirm the regioselectivity of the reactions. Further substitution at the boron atom produced nona-functionalized BODIPYs 7a,b, which show 676 and 739 nm emissions with 91 and 100 nm Stokes shifts, respectively.
 Please wait while we load your content...
Please wait while we load your content...