Chemoenzymatic synthesis and utilization of a SAM analog with an isomorphic nucleobase†
Abstract
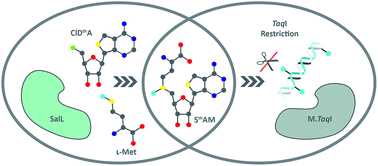
SalL, an enzyme that catalyzes the synthesis of SAM from L-methionine and 5′-chloro-5′-deoxyoadenosine, is shown to accept 5′-chloro-5′-deoxythienoadenosine as a substrate and facilitate the synthesis of a synthetic SAM analog with an unnatural nucleobase. This synthetic cofactor is demonstrated to replace SAM in the DNA methylation reaction with M.TaqI.
 Please wait while we load your content...
Please wait while we load your content...