The squaramide-catalyzed asymmetric Michael/cyclization tandem reaction for the synthesis of chiral trifluoromethylated hydroxyimino tetrahydrobenzofuranones†
Abstract
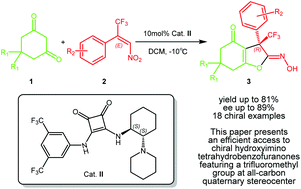
An enantioselective synthesis of trifluoromethylated hydroxyimino tetrahydrobenzofuranones has been developed. 1,3-Dicarbonyl carbocyclic compounds react with β-CF3-β-disubstituted nitroalkenes in the presence of a chiral squaramide catalyst, providing efficient access to diverse hydroxyimino tetrahydrobenzofuranones featuring a trifluoromethyl group at the C3-position of an all-carbon quaternary stereocenter with good yields (up to 81%) and enantioselectivities (up to 89% ee).

 Please wait while we load your content...
Please wait while we load your content...