Maltotriose-conjugation to a fluorinated chlorin derivative generating a PDT photosensitizer with improved water-solubility†
Abstract
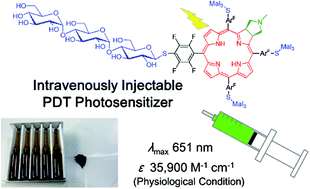
Photoactive molecules with the frameworks of chlorin and/or porphyrin possessing four perfluorinated aromatic rings were conjugated with maltotriose (Mal3) via the nucleophilic aromatic substitution reaction and subsequent deprotection reaction of the oligosaccharide moieties. The resulting oligosaccharide-conjugated molecules are ultimately improved as compared to the previously reported monosaccharide-counterparts in terms of water-solubility. In particular, a water-soluble chlorin derivative surrounded by four Mal3 molecules showed an excellent biocompatibility, strong photoabsorption in the longer wavelength regions, and a very high photocytotoxicity. Thus, the present synthetic route combined with the use of an oligosaccharide was shown to be a straightforward strategy to develop a third generation photosensitizer for photodynamic therapy (PDT).

 Please wait while we load your content...
Please wait while we load your content...