Abstract
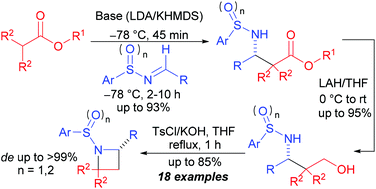
A simple and efficient synthetic route to substituted N-sulfinyl and N-sulfonyl azetidines is described involving imino-aldol reaction of ester enolates with racemic and non-racemic aldimines for obtaining β-amino esters as a key step. These β-amino esters on subsequent reduction followed by TsCl/KOH mediated cyclization produced the corresponding racemic and non-racemic azetidines with high yield and stereoselectivity.

 Please wait while we load your content...
Please wait while we load your content...