A fluorescence study of isofagomine protonation in β-glucosidase†
Abstract
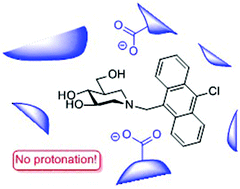
N-(10-Chloro-9-anthracenemethyl)isofagomine 5 and N-(10-chloro-9-anthracenemethyl)-1-deoxynojirimycin 6 were prepared, and their inhibition of almond β-glucosidase was measured. The isofagomine derivative 5 was found to be a potent inhibitor, while the 1-deoxynojirimycin derivative 6 displayed no inhibition at the concentrations investigated. Fluorescence spectroscopy of 5 with almond β-glucosidase at different pH values showed that the inhibitor nitrogen is not protonated when bound to the enzyme. Analysis of pH inhibition data confirmed that 5 binds as the amine to the enzyme's unprotonated dicarboxylate form. This is a radically different binding mode than has been observed with isofagomine and other iminosugars in the literature.

 Please wait while we load your content...
Please wait while we load your content...