Design and synthesis of new fluconazole analogues†
Abstract
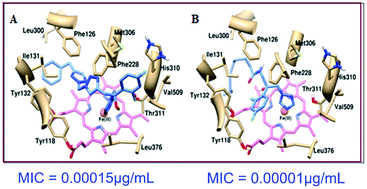
We have synthesized new fluconazole analogues containing two different 1,2,3-triazole units in the side chain. The synthesis of new amide analogues using a variety of acids is also described. All the compounds showed very good antifungal activity. A hemolysis study of the most active compounds 6e and 13j showed that both compounds did not cause any hemolysis at the dilutions tested. These compounds did not exhibit any toxicity to L929 cells at MIC and lower concentrations. In the docking study, the overall binding mode of 6e and 13j appeared to be reasonable and provided a good insight into the structural basis of inhibition of Candida albicans Cyp51 by these compounds.

 Please wait while we load your content...
Please wait while we load your content...