Selective aliphatic carbon–hydrogen bond activation of protected alcohol substrates by cytochrome P450 enzymes†
Abstract
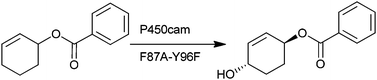
Protected cyclohexanol and cyclohex-2-enol substrates, containing benzyl ether and benzoate ester moieties, were designed to fit into the active site of the Tyr96Ala mutant of cytochrome P450cam. The protected cyclohexanol substrates were efficiently and selectively hydroxylated by the mutant enzyme at the trans C–H bond of C-4 on the cyclohexyl ring. The selectivity of oxidation of the benzoate ester protected cyclohexanol could be altered by making alternative amino acid substitutions in the P450cam active site. The addition of the double bond in the cyclohexyl ring of the benzoate ester protected cyclohex-2-enol has a debilitative effect on the activity of the Tyr96Ala mutant with this substrate. However, the Phe87Ala/Tyr96Phe double mutant, which introduces space at a different location in the active site than the Tyr96Ala mutant, was able to efficiently hydroxylate the C–H bonds of 1-cyclohex-2-enyl benzoate at the allylic C-4 position. Mutations at Phe87 improved the selectivity of the oxidation of 1-phenyl-1-cyclohexylethylene to trans-4-phenyl-ethenylcyclohexanol (92%) when compared to single mutants at Tyr96 of P450cam.

 Please wait while we load your content...
Please wait while we load your content...