The effect of heteroatom conformation on optoelectronic properties of cyclopentadithiophene derivatives†
Abstract
Cyclopentadithiophene (CPDT) derivatives with different heteroatom conformations have been synthesized. The optical, electrochemical and charge transport properties of these molecules are reported. The CPDT-anti-ketone not only exhibits the lowest optical and electronic bandgaps, but also exhibits reasonable hole mobility, 3 × 10−3 cm2 (V s)−1. Changing the carbonyl conformation to the syn position or incorporating the imine functionality results in a blue-shift in the lower energy band of the absorption spectrum indicative of the increased bandgaps.
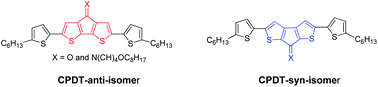

 Please wait while we load your content...
Please wait while we load your content...