Reaction site-driven regioselective synthesis of AChE inhibitors†
Abstract
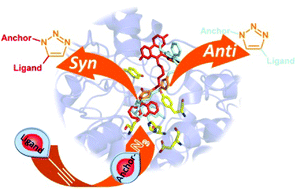
The enzyme-directed synthesis is an emerging fragment-based lead discovery approach in which the biological target is able to assemble its own multidentate ligands from a pool of building blocks. Here, we report for the first time the use of the human acetylcholinesterase (AChE) as an enzyme for the design and synthesis of new potent heterodimeric huprine-based inhibitors. Both the specific click chemistry site within the protein and the regioselectivity of the Huisgen cycloaddition observed suggest promising alternatives in the design of efficient mono- and dimeric ligands of AChE. Finally, a detailed computational modelling of the click reaction was conducted to further understand the origin of this TGS selectivity.

 Please wait while we load your content...
Please wait while we load your content...