One-pot formation of fluorescent γ-lactams having an α-phosphorus ylide moiety through three-component α(δ′)-Michael reactions of phosphines with an enyne and N-tosyl aldimines†
Abstract
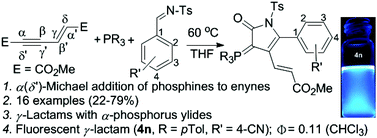
We demonstrate a straightforward synthesis of γ-lactams possessing an α-phosphorus ylide moiety from assembly of phosphines, N-tosyl aldimines and an enyne through an initial α(δ′)-attack of phosphines to an enyne in up to 79% yield. The investigated multicomponent reaction tolerates a variety of triarylphosphines and electron-poor aldimines to give γ-lactams in one pot. One of the lactams, with the tri(p-tol)phosphine and 4-cyanophenyl moiety, exhibits fluorescence emission at 447 nm with a quantum yield of 0.11.

 Please wait while we load your content...
Please wait while we load your content...