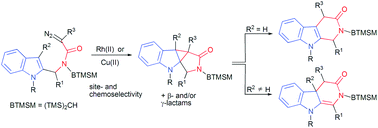
The Rh(II)- and Cu(II)-catalyzed reactions of N-bis(trimethylsilyl)methyl, N-(2-indolyl)methyl α-diazoamides are investigated to delineate how conformational, steric and electronic factors influence the site- and chemoselectivity of the metallocarbenoid reaction. The N-bis(trimethylsilyl)methyl (N-BTMSM) group is found to be essential in promoting the metallocarbenoid reaction at the N-(2-indolyl)methyl moiety as well as providing subtle but effective conformational influence about the amide N–Cα sigma bond in diazoamides carrying an N–Cα alkoxymethyl side-chain, to afford excellent site- and chemoselectivity. In general, the metal-catalyzed reactions are found to favor metallocarbenoid addition to the indole C(2)–C(3) double bond over C–H insertion to give cyclopropanated products (tetracyclic γ-lactams); however, chemoselectivity is also affected by steric effects, as revealed in the N-[2-(3-methylindolyl)]methyl diazoamides, and to some extent by the nature of the catalyst employed, as seen in the N–Cα-alkoxymethyl diazoamides. The tetracyclic γ-lactams are found to rearrange to give good to high yields of the tricyclic indole derivatives under the metallocarbenoid reaction conditions or under acidic conditions. The propensity of the tetracyclic γ-lactams to undergo rearrangement is found to be dependent on the nature of the α-substituent on the original diazo carbon and the indole N-substituent.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...