Intra- and intermolecular complexation in C(6) monoazacoronand substituted cyclodextrins†
Abstract
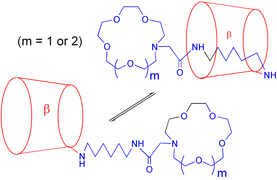
The preparation of 6A-deoxy-6A-(6-(2-(1,4,7,10-tetraoxa-13-azacyclopentadecan-13-yl)acetamido)hexylamino)-α-cyclodextrin, 3, 6A-deoxy-6A-(6-(2-(1,4,7,10,13-pentaoxa-16-azacyclooctadecan-16-yl)acetamido)hexylamino)-α-cyclodextrin, 4, and their β-cyclodextrin analogues, 5 and 6, are described. 1H (600 MHz) ROESY NMR spectra of the C(6) substituted β-cyclodextrins, 5 and 6, are consistent with the intramolecular complexation of their azacyclopentadecanyl- and azacyclooctadecanyl(acetamido)hexylamino substituents in the β-cyclodextrin annulus in D2O at pD = 8.5 whereas those of their α-cyclodextrin analogues, 3 and 4 are not complexed in the α-cyclodextrin annulus. This is attributed to the monoazacoronand components of the substituents being able to pass through the β-cyclodextrin annulus whereas they are too large to pass through the α-cyclodextrin annulus. However, the substituents of 3 and 4 are intermolecularly complexed by β-cyclodextrin to form pseudo [2]-rotaxanes. Metallocyclodextrins are formed by 5 through complexation by the monoazacoronand substituent component for which log (K/dm3 mol−1) = <2, 6.34 and 5.38 for Ca2+, Zn2+ and La3+, respectively, in aqueous solution at 298.2 K and I = 0.10 mol dm−3 (NEt4ClO4).

 Please wait while we load your content...
Please wait while we load your content...