Design and synthesis of selective, small molecule inhibitors of coactivator-associated arginine methyltransferase 1 (CARM1)†‡
Abstract
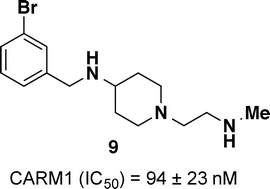
Coactivator-associated arginine methyltransferase 1 (CARM1) is a type I protein arginine methyltransferase (PRMT) that catalyzes the conversion of arginine into monomethylarginine (MMA) and further into asymmetric dimethylarginine (ADMA). CARM1 methylates histone 3 arginines 17 and 26, as well as numerous non-histone proteins including CBP/p300, SRC-3, NCOA2, PABP1, and SAP49, while also functioning as a coactivator for various proteins that have been linked to cancer such as p53, NF-κβ, β-catenin, E2F1 and steroid hormone receptor ERα. As a result, CARM1 is involved in transcriptional activation, cellular differentiation, cell cycle progression, RNA splicing and DNA damage response. It has been associated with several human cancers including breast, colon, prostate and lung cancers and thus, is a potential oncological target. Herein, we present the design and synthesis of a series of CARM1 inhibitors. Based on a fragment hit, we discovered compound 9 as a potent inhibitor that displayed selectivity for CARM1 over other PRMTs.

 Please wait while we load your content...
Please wait while we load your content...