Tuning the dipolar second-order nonlinear optical properties of 5-π-delocalized-donor-1,3-di(2-pyridyl)benzenes, related cyclometallated platinum(ii) complexes and methylated salts†
Abstract
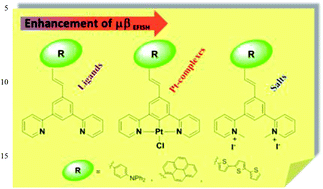
The synthesis and characterization of three 5-π-delocalized-donor-1,3-di(2-pyridyl)benzenes is reported along with that of their related cyclometallated platinum(II) complexes and N,N-dimethylated iodide salts. The second-order nonlinear optical (NLO) properties of all the compounds have been determined by the Electric Field Induced Second Harmonic generation technique, showing how the μβEFISH absolute value of 1,3-di(2-pyridyl)benzenes can be tuned by the nature of the substituent on position 5 of the central benzene ring, and greatly increased by cyclometallation to Pt or by N-methylation.


 Please wait while we load your content...
Please wait while we load your content...