Water-soluble Schiff base-actinyl complexes and their effect on the solvent extraction of f-elements†
Abstract
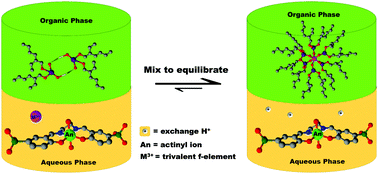
Conventional solvent extraction of selected f-element cations by bis(2-ethylhexyl)phosphoric acid (HDEHP) yields increased extraction from aqueous to organic solution along the series Np(V) < Cm(III) < Eu(III) < U(VI), with distribution ratios all within two orders of magnitude. However, in the presence of the water-soluble tetradentate Schiff base (N,N′-bis(5-sulfonatosalicylidene)-ethylenediamine or H2salenSO3), selective complexation of the two actinyl cations (Np(V) and U(VI)) resulted in an extraction order of Np(V) < U(VI) ≪ Eu(III) < Cm(III). The extraction of neither Cm(III) or Eu(III) by HDEHP are significantly impacted by the presence of the aqueous phase Schiff base. Despite observed hydrolytic decomposition of H2salenSO3 in aqueous solutions, the calculated high conditional stability constant (β11 = 26) for the complex [UO2(salenSO3)]2− demonstrates its capacity for aqueous hold-back of U(VI). UV-visible-NIR spectroscopy of solutions prepared with a Np(VI) stock and H2salenSO3 suggest that reduction of Np(VI) to Np(V) by the ligand was rapid, resulting in a pentavalent Np complex that was substantially retained in the aqueous phase. Results from 1H NMR of aqueous solutions of H2salenSO3 with U(VI) and La(III), Eu(III), and Lu(III) provides additional evidence that the ligand readily chelates U(VI), but has only weak interactions with trivalent lanthanide ions.

 Please wait while we load your content...
Please wait while we load your content...