Octahedral molybdenum cluster complexes with aromatic sulfonate ligands†
Abstract
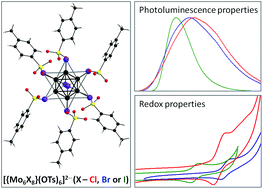
This article describes the synthesis, structures and systematic study of the spectroscopic and redox properties of a series of octahedral molybdenum metal cluster complexes with aromatic sulfonate ligands (nBu4N)2[{Mo6X8}(OTs)6] and (nBu4N)2[{Mo6X8}(PhSO3)6] (where X− is Cl−, Br− or I−; OTs− is p-toluenesulfonate and PhSO3− is benzenesulfonate). All the complexes demonstrated photoluminescence in the red region and an ability to generate singlet oxygen. Notably, the highest quantum yields (>0.6) and narrowest emission bands were found for complexes with a {Mo6I8}4+ cluster core. Moreover, cyclic voltammetric studies revealed that (nBu4N)2[{Mo6X8}(OTs)6] and (nBu4N)2[{Mo6X8}(PhSO3)6] confer enhanced stability towards electrochemical oxidation relative to corresponding starting complexes (nBu4N)2[{Mo6X8}X6].


 Please wait while we load your content...
Please wait while we load your content...